Sideritis spp. Extracts Enhance Memory and Learning in Alzheimer’s β-Amyloidosis Mouse Models and Aged C57Bl/6 Mice
Abstract
Nowadays, Alzheimer’s disease is the most prevalent epiphenomenon of the aging population. Although soluble amyloid-β (Aβ) species (monomers, oligomers) are recognized triggers of the disease, no therapeutic approach is able to stop it. Herbal medicines are used to treat different diseases in many regions of the world. On the Balkan Peninsula, at the eastern Mediterranean Sea, and adjacent regions, Sideritis species are used as traditional medicine to prevent age-related problems in elderly. To evaluate this traditional knowledge in controlled experiments, we tested extracts of two commonly used Sideritis species, Sideritis euboea and Sideritis scardica, with regard to their effects on cognition in APP-transgenic and aged, non-transgenic C57Bl/6 mice. Additionally, histomorphological and biochemical changes associated with Aβ deposition and treatment were assessed. We found that daily oral treatment with Sideritis spp. extracts highly enhanced cognition in aged, non-transgenic as well as in APP-transgenic mice, an effect that was even more pronounced when extracts of both species were applied in combination. The treatment strongly reduced Aβ42 load in APP-transgenic mice, accompanied by increased phagocytic activity of microglia, and increased expression of the α-secretase ADAM10. Moreover, the treatment was able to fully rescue neuronal loss of APP-transgenic mice to normal levels as seen in non-transgenic controls. Having the traditional knowledge in mind, our results imply that treatment with Sideritis spp. extracts might be a potent, well-tolerated option for treating symptoms of cognitive impairment in elderly and with regard to Alzheimer’s disease by affecting its most prominent hallmarks: Aβ pathology and cognitive decline.
INTRODUCTION
To date, the main targets for therapeutic intervention of Alzheimer’s disease (AD) are (i) the inhibition of amyloidogenic amyloid-β protein precursor processing to reduce amyloid-β (Aβ) production and (ii) the resolution of Aβ aggregates or even plaques [1–3]. In this regard, a continuously growing number of substances from botanical sources are under consideration to have beneficial effects on AD pathology. St. John’s wort (Hypericum perforatum) [4, 5], green tea (Camellia sinensis) [6–8], polyphenols of red wine [9], and the ayurvedic Ashwanganda (Withania somnifera) [10] were found to reduce Aβ deposition and enhance cognition. Nonetheless, large-scale studies failed to prove prevention of dementia or reduction of clinical progress in patients using formerly promising Ginkgo biloba extracts [11, 12]. Since the pathogenic mechanisms for AD are still under discussion [13], it seems difficult to specifically guide the search for new effective substances and treatment strategies.
We decided to make use of handed-down knowledge and searched for plants 1) long-known in traditional medicine for their effects on cognition or 2) which are said to somehow reduce mental problems in the local communities. Often designated as ‘longevity agents’ (ethno-medicinal approach) since used mostly by elderly and oldest-old with mental problems, these plants grow naturally in their geographic regions of traditional use. Species of the genus Sideritis spp. are members of the Lamiaceae family and are mostly unknown in western academic medicine. The genus Sideritis consists of approximately 140 species and is primarily distributed throughout the Balkan Peninsula and Mediterranean region [14]. Some species are found naturally also in Hungary (Sideritis montana) and as cultivated plants as far north as in Tromsø/Norway (Sideritis scardica). Particularly the Greek species, Sideritis euboea and Sideritis scardica, known as Greek mountain tea (τσαι τoυ βoυνoυ), have a long history in traditional Mediterranean medicine, yet most of the medical uses of Sideritis spp. are limited to traditional medicine [15]. Nowadays, the herbs are known to enhance the antioxidant defense of the adult rodent brain and to act anti-microbiologically [16]. Newer investigations demonstrated that water and alcoholic extracts of S. scardica inhibited the serotonin, noradrenaline, and dopamine uptake in rat brain synaptosomes in a dose depended manner [17]. Detailed influence of the different extraction solvents on the efficiency of S. scardica extracts in the same model were also reported in [18]. In case of the use of water as traditional extracting agent (tea preparation), 3 to 6-fold lower IC50-values compared to hydro-alcoholic extracts have been measured. Sideritis species are also renowned to be a rich source of a variety of flavonoids [15, 19]. Studies suggest that their secondary metabolites are able to improve memory capacity by enhancing the efficiency of information storage and retrieval [20, 21]. There is also increasing evidence that flavonoid-rich foods such as fruit juices and red wine or supplements might delay the initiation of neurodegenerative disorders such as AD or slow down their progression [22]. Other studies showed that flavonoids bear anti-inflammatory abilities accompanied by increased Aβ phagocytosis by microglia and macrophages in vitro [23].
Here, we report the first scientific evaluations for the in vivo effectiveness of extracts of two Sideritis species, S. euboea and S. scardica Griseb., in treating cognitive decline in a rodent model of AD and in aged, non-transgenic C57Bl/6 mice. We have screened individual extracts as well as an extract combination of both species with regard to their effects on morphological and biochemical hallmarks of AD, the accompanying neuronal loss, and their ability to delay the deterioration of cognition and memory. We demonstrate that the single extracts, and even more efficiently the combination of both, improve memory performance and counteract neurodegeneration in vivo in aged non-transgenic as well as in APP-transgenic mice. Moreover, daily oral administration of Sideritis extracts was highly effective in decreasing intracerebral Aβ42 levels and the amount of amyloid plaques, probably by induction of ADAM10 expression and stimulation of microglial response. We conclude that Sideritis extracts, beside their attenuating effects in AD proteopathy, also have a positive effect on cognitive performance in healthy, elderly animals. Hence, our work reveals strong memory enhancing properties of Sideritis scardica andS. euboea extracts in experimental settings, supporting their traditional use as potent and compliant treatment in elderly or as preventive option for dementing disorders like AD.
METHODS
Animals and treatment
Transgenic mice, which express mutated human amyloid precursor protein (APP) and presenilin 1 (PS1) under control of the Thy1-promoter (APPKM670/671NL, PSL166P) in the C57Bl6/J background [24], were generously provided by the University of Tübingen (Germany) and are hereafter referred to as ‘APP-tg’ mice. Dry extract powders re-suspended in water in addition to a ‘water only’ vehicle control were applied by daily gavage at the age of 40 days (AD-initiation) or 50 days (post-AD-onset), respectively, up to the age of 100 days.
The species Sideritis scardica and Sideritis euboea as well as a 1:1 combination of both extracts were applied in two dosages: 1.2 g/kg body weight (AD-initiation) and 12 g/kg body weight (post-AD-onset).
Non-transgenic mice were treated for 15 days (short-term) with an intermediate dosage of 6 g/kg body weight of the Sideritis extract combination, starting at an age of 135 days for 15 consecutive days until an age of 150 days (first period), and repeatedly for three further periods of 15 days at ages 300, 450, and 600 days, respectively.
The weight of treated mice was measured daily for reasons of dosage and health monitoring; numbers of animals used are indicated in Table 1. All mice were housed in 12-h day/night cycles at 21–22°C with free access to food and water. All experiments were approved by the local animal ethics committee (LALLF) and carried out according to state law of Mecklenburg-Western Pomerania, Germany.
Plant extract preparation
Extracts of Sideritis scardica and Sideritis euboea as well as the extract combination were produced by Finzelberg GmbH & Co. KG (Andernach, Germany) in order to ensure quality and purity at pharmacological standards. The plants were harvested during flowering season between June and August and got dried to a residual humidity of < 12%. The raw herb material was cut to 1-2 cm pieces and extracted exhaustively using ethanol 20% (v/v) in an industrial percolator. The solvent was gently removed from the extracts via evaporation in vacuo up to a viscous soft extract. Afterwards, drying excipient maltodextrin was added and the complete extract preparation (70% native extract, 30% maltodextrin) was pasteurized and finally dried under gentle conditions. The resulting dry extracts were milled to a fine powder (Table 1). For the purpose of administration, extracts were re-dissolved in tap water.
Tissue preparation
As previously described [4, 25], mice were sacrificed by cervical dislocation and transcardially perfused with PBS. One hemisphere was fixated using 4% paraformaldehyde in PBS for immunohistochemistry. The other hemisphere was snap-frozen in liquid nitrogen and stored at –80°C for later biochemical analyses. Frozen hemispheres were homogenized using a bead homogenizer (PreCellys®24, Peqlab, Germany) for immediate biochemical analysis.
Enzyme-linked immunosorbent assay (ELISA)
Intracerebral Aβ42 levels were determined with an Aβ42-specific ELISA Kit (TK42HS, The Genetics Company, Schlieren, Switzerland) By using two buffers, two protein fractions were extracted as described before [4, 26]: (i) the carbonate buffer-soluble fraction containing soluble Aβ species and (ii) the guanidine hydrochloride fraction containing aggregated Aβ species. ELISAs were performed according to the manufacturer’s instructions using appropriate dilutions. The total protein content was measured in triplicate with the help of a NanoDrop 1000 at 280 nm (Thermo Fisher Scientific, USA).
Immunohistochemistry
Brains were fixated and prepared as described [4, 25, 27]. Sections were stained with BOND-MAX (Menarini/Leica, Germany) automated immunostaining system for anti-human Aβ clone 6F3D (1:200, Dako) and the BondTM Polymer Refine Detection kit (Menarini/Leica, Germany). Double-stained sections for Aβ and microglia were immunostained in a second step using anti-Iba1 (1:1,000, Wako) and the BondTM Polymer AP-Red detection kit (Menarini/Leica, Germany). Neurons were stained with anti-NeuN antibody (1:1,000, Millipore). Tissue sections were digitized at 230 nm resolution with the MiraxMidi Slide Scanner (ZeissMicroImaging GmbH, Germany) and semi-automatically analyzed by the AxioVision software package (ZeissMicroImaging GmbH, Germany) [28, 29].
Semi-automated analyzes of brain slices
Cortical regions of interest (ROIs) were defined and stained structures (plaques, microglia, or neurons, respectively) were segregated according to their RGB color profile using the appropriate color channels. The resulting binary pictures were digitally processed to gain the shape corresponding to the initial scan. Plaque/microglia double staining analyses were performed as published in [28, 29]. Plaque-associated microglial area and the number of plaques covered more than 50% by microglia have been determined. For plaque analyses, the plaque number and sizes were quantified across all cortical layers. The obtained data (i.e., ROI size, plaque number, plaque area) were recorded, plaque coverage was calculated as percentage of stained area versus ROI, and all data were finally normalized to 10 mm2 for each hemisphere to control for ROI differences. For the analyses of number of neurons, the NeuN-stained area was measured and calculated as percentage of the cortical ROI, normalized to 10 mm2, thus, representing the proportion of neurons in the cortex.
Morris water maze
For behavioral analyses of the cognitive abilities of the APP-tg mice, a Morris water maze protocol adapted from the Johns Hopkins Neurogenetics and Behavior Center was used as described [4, 30]. In brief, mice were trained and tested in two sessions per day over a period of four consecutive days. Both sessions were separated by four hours and consisted of four trials per session. Few protocol modifications were made to test the extract’s influence on the cognitive abilities of aged, non-tg mice after a standard initial test procedure at the age of 150 days. To evaluate long-term memory at the ages 300, 450, and 600 days, mice firstly had to pass the standard platform position learned 150 days ago (day one, first session). Subsequently, a new escape platform position was taught (day one, second session) and retrieved in all following sessions of this training period. Thus, the test not only assessed long-term memory, but also the mice’ ability to relearn against previously acquired local orientation when being confronted with a new platform position (re-orientation).
Western blot
Cerebral tissue homogenates were fractionated into three protein fractions according to Lesné et al. [31]. After SDS-PAGE using 10μg total protein per lane, proteins were blotted onto a PVDF membrane. Blots were probed for ADAM10 (1:1,000, Calbiochem, Germany), BACE1 (1:1,000, Abcam, USA), or β-actin (1:20,000, Sigma-Aldrich, Germany) dissolved in Odyssey® blocking buffer (LI-COR, USA). IRDye® secondary anti-mouse, anti-rat, and anti-rabbit antibodies (all diluted 1:10,000, LI-COR, USA) were used as detection antibodies. Target proteins were visualized and quantified with the help of the Odyssey® two-channel IR detection system (LI-COR, USA). Total protein concentrations were determined using a BCAtrademark protein assay kit (Pierce, part of Thermo Fisher Scientific, Rockford, USA).
ABC-transporter activity assay
In vitro ABCB1 and ABCC1 activities were mea-sured using the SB MDR1 and MRP1 PREDEA-SYTM ATPase Kits (Solvo Biotechnology, Budapest, Hungary), respectively, according to the manufacturer’s instructions. Sideritis spp. extracts were diluted in DMSO (Sigma-Aldrich, Germany) to a final DMSO concentration of 0.05μg/ml and activity was measured in 96-well plates using a Paradigm spectrophotometer (Beckman Coulter, Germany) at 610 nm.
Statistical analysis
Analyses and behavioral tests were made without prior knowledge of the experimental group. Results are presented as means + standard error of the mean (SEM). The corresponding numbers of laboratory animals can be found in Table 1. Significance was calculated versus vehicle-treated controls by using one-way ANOVA/Students t-Test (as appropriate) with Holm-Sidak’s correction for multiple comparisons. Values with a probability level of less than 0.05 (p < 0.05) were regarded as significant. All statistical calculations were performed using the GraphPad Prism 5 software (GraphPad Software Inc., LaJolla, USA).
RESULTS
Sideritis spp. extracts rescue cognitive performance in APP-tg mice
Due to the reported vitalizing effect of different Sideritis species [14, 32], the effects of two potent species of this genus on spatial memory function in APP-tg mice were evaluated by Morris water maze. Both single extracts showed no improvement of spatial memory in the AD-initiation treatment paradigm. In contrast, the extract combination of both resulted in a significant memory enhancement shown by escape latencies reduced by 59% (day 3) and 46% (day 4) compared to vehicle-treated littermates. Of note, these values are similar to vehicle-treated, non-transgenic mice (Fig. 1A). Using the post-AD-onset treatment paradigm the S. euboea extract increased the memory performance by 40% at day 4 in comparison with vehicle-treated mice (Fig. 1B). Again, treatment with the extract combination proofed to be more effective and already reduced the escape latencies at day 2 by 55% and more marked by 60% (day 3), and 61% (day 4) on the consecutive days when compared to the vehicle-treated control group.
Treatment with Sideritis spp. leads to a strong decrease of soluble Aβ42 and protects from neuronal decline
In 100-days-old mice, AD-initiation therapy reduced intracerebral, buffer-soluble Aβ42 levels significantly by 42% (S. scardica), 40% (S. euboea), and 40% (combination) in comparison to vehicle-treated littermates (Fig. 2A). Similarly, a treatment using the post-AD-onset paradigm significantly decreased soluble Aβ42 levels by 58% (S. scardica), 60% (S. euboea), and 56% (combination). Interestingly, no significant reduction of guanidine-soluble Aβ42 could be detected in any paradigm (not shown). Based on these results, we determined the influence of the extracts on neuronal cell loss to indicate potential neuroprotective properties. To do so, we used anti-NeuN-stained brain slices of 100-days-old mice and quantified the NeuN-positive area within the whole cortex. Quantifications unveiled a significant neuron loss by 16% in vehicle-treated APP-tg mice in comparison to vehicle-treated, non-transgenic mice in both paradigms (Fig. 2B). Intriguingly, AD-initiation treatment rescued neuronal cells as indicated by a significant increase in neuronal area as compared to vehicle-treated APP-tg mice by 27% (combination) and 32% (S. euboea). Moreover, post-AD-onset treatment with the extract combination significantly increased neuronal area by 50% in comparison to vehicle-treated APP-tg littermates (Fig. 2B–D).
Sideritis spp. extracts decrease Aβ depositions
We analyzed brain slices stained immunohistochemically for Aβ (6F3D; Fig. 3A–D) to evaluate the effect of the Sideritis spp. extracts on Aβ deposition and plaque formation. Comparison of morphological plaque burden between control mice and the AD-initiation treatment group revealed no significant changes regarding plaque numbers (Fig. 3E). However, AD-initiation treatment using the extract combination led to a significant 26% decrease of the mean plaque size (Fig. 3F). Contrary, post-AD-onset treatment with either extract or their combination decreased plaque numbers by 32% (S. scardica), 41% (S. euboea), and 42% (combination), respectively (Fig. 3G). Additionally, the plaque size was reduced by 31% (S. scardica), 36% (S. euboea), and 25% (combination) (Fig. 3H).
Sideritis spp. extracts activate and intensify Aβ phagocytic microglia
It has been reported that microglia are closely connected with soluble Aβ load and its depositions [33, 34]. To determine possible phagocytosis-enhancing properties of Sideritis spp. extracts on microglia localization in vivo, we performed immunohistochemical co-staining against both Aβ (6F3D) and microglial cells (Iba-1), on paraffin-embedded brain sections (Fig. 4A–D). Computer-assisted analyses [28] of the cortical microglia area in the vicinity of Aβ deposits revealed significantly enhanced microglia response after extract application in the AD-initiation paradigm. Microglia area was increased by 107% (S. scardica), 111% (S. euboea), and 139% (combination) in the vicinity of plaques (Fig. 4E). Moreover, post-AD-onset treatment showed an augmentation of the cortical microglia area by 150% (S. scardica), 138% (S. euboea), and 181% (combination) (Fig. 4G). In addition, the relative amount of plaques covered by more than 50% with microglia was analyzed. However, no change was found after any treatment paradigm (Fig. 4H).
Sideritis spp. extracts enhance ADAM10 expression
Protein expression of the most Aβ-relevant secretases, the α- (ADAM10) and β-secretase (BACE1), was determined by western blot analyses to reveal whether Sideritis spp. extracts are able to alter the balance of AβPP processing enzymes (Fig. 5). Since the γ-secretase is stabile driven by the PS1-transgene expression in the used mouse model, it has not been analyzed [24]. Quantification of proteinexpression levels indicated that administration according to the AD-initiation and post-AD-onset paradigms significantly increased ADAM10 expression. Even more interesting, ADAM10 expression was 3.5-fold increased after post-AD-onset treatment with the combination extract of both species. We found only minor changes of the BACE1 protein expression after AD-initiation but not after post-AD-onset treatment (Fig. 5).
Extract combination of Sideritis spp. prevents progress of Aβ42 augmentation
To further understand the effect elicited by Sideritis spp. Treatment, we analyzed the brain content of soluble Aβ42 of treated and untreated 75-days-old APP-tg mice. We detected a significant decrease of soluble Aβ42 levels already at this age in the AD-initiation paradigm as well as the post-AD-onset treatment with all extracts by about 40% (data not shown). Next, we calculated the fold-difference of buffer-soluble Aβ42 at 100 d of age for the different treatment groups compared to untreated 75-days-old naïve animals. Interestingly, the calculation for the extract-treated, post-onset groups revealed that the Sideritis spp. extract treatment stabilizes the amount of buffer-soluble Aβ42 at the level of 75-days-old untreated animals, while it was 2-fold higher in vehicle-treated mice (Fig. 6).
Extract combination of Sideritis spp. highly enhances cognitive performance in aging non-tg mice
Based on our results, we asked whether an extract combination of both Sideritis scardica and Sideritis euboea might have improving effects on cognitive functions even in “healthy” aging mice (C57Bl/6). Thus, we analyzed non-tg mice in the Morris water maze test to compare spatial memory and relearning ability with the Sideritis spp. extract combination and vehicle-treated littermates. The escape latency was significantly reduced by 54% (day 2), 49% (day 3), and 60% (day 4) in the treated animals, pointing to an improved cognitive performance in comparison to vehicle-treated mice (Fig. 7A). This application mode and test procedure was then repeated with new platform positions at the ages of 300, 450, and 600 days, respectively (Fig. 7B, C). We observed a significant memory improvement in each learning period as revealed by a significant decrease in escape latencies at the last test day (day 4) of each period by 62% (300 d), 50% (450 d), and 50% (600 d) upon Sideritis spp. extract combination treatment. In addition, we evaluated the capacity to adapt to and to memorize new information represented by an altered platform position (Fig. 7D). To do so, we compared the escape latencies measured with the old and new platform positions. The results showed significantly reduced escape latencies by 54% (150 d), 55% (300 d), 54% (450 d), and 66% (600 d) as compared to vehicle-treated mice, which indicated a highly enhanced ability to learn and retrieve new information due to Sideritis spp. extract application.
DISCUSSION
While soluble Aβ peptides are highly neurotoxic [35–38], aggregated forms act on network communication [39] and occur in a variety of conditions, accompanied by dystrophic neurites and disrupted axonal transport [40, 41]. Not only, but especially the soluble Aβ concentration can be a predictor of synaptic change and cognitive decline and determines the severity of neurodegeneration and memory disruption [42–44]. Additionally, high levels of soluble Aβ assemblies elicit aberrant excitatory activity in cortical-hippocampal networks and disrupt theneuronal network communication [39, 45]. Using two treatment paradigms, an AD-initiation and a post-AD-onset strategy, we were able to confirm these results and define treatment options that attenuate and even rescue the cognitive capacity in the APP-tg mouse model. Sideritis spp. given as post-AD-onset treatment prevented the progress of toxic Aβ42 augmentation. As visualized in Fig. 6, treatment from an age of 50 d stabilized the amount of soluble Aβ42 until an age of 100 d at the mean level of 75-days-old, non-treated animals. These findings highly correlate with the improved cognitive performance verified by Morris water maze.
However, in the present study the correlation between plaque burden and memory loss during AD pathogenesis was much higher and also dose dependent in the case of Sideritis monospecies extracts. High-dose, post-AD-onset, single extract treatment not only reduced buffer-soluble Aβ42, but also significantly decreased the plaque burden, which was accompanied by memory performances rescued to levels of non-transgenic littermates, whereas low-dose treatment only had an effect on buffer-soluble Aβ42 levels. In contrast, using the extract combination, both treatment paradigms consistently showed rescued memory and reduction of plaque burden in APP-tg mice.
Sideritis spp. are renowned to be a rich source of a variety of flavonoids [15, 19]. Several studies indicate that flavonoid consumption may be capable of inducing improvements in cognitive performance particularly because of its anti-inflammatory properties [46–48]. Recent evidence has shown that this group of plant-derived compounds may exert particularly powerful actions on mammalian cognition and may reverse age-related declines in memory and learning [21]. Furthermore, Qin et al. indicated that polyphenols of green tea have a protective effect on cultured rat primary prefrontal cortical neurons against Aβ-induced cytotoxicity [49]. Our analyses of brain sections stained for the neuronal marker protein NeuN showed that the treatment of APP-tg mice with Sideritis spp. extracts led to a significant decrease of neuronal loss in comparison to vehicle treated APP-tg mice. Especially the extract combination as well as the S. euboea only extract had strong neuroprotective effects. Moreover, Sideritis spp. treated APP-tg mice had a neuronal density comparable to that of vehicle-treated, non-transgenic mice. This is the first scientific report that Sideritis species extracts display a persuasive neuroprotective potential after oral treatment.
The response of microglia to senile plaques in AD is not fully understood. Although acute inflammatory stimuli induce beneficial effects, such as tissue repair processes and Aβ phagocytosis, uncontrolled and chronic inflammation may result in production of neurotoxic factors that amplify underlying disease states and lead to neuronal death [50, 51]. It is known that soluble Aβ-species and aggregated proteins are able to trigger microglial activity[52, 53]. We have shown earlier that microglia seem to respond to small, probably young plaques very fast and consequently, whereas the attraction to or activity at larger plaques is less distinct [27]. If this is due to a different composition of larger plaques, a microglial “shut off” or phenotype switch after prolonged contact to a plaque or other mechanisms needs to be resolved. Our analyses show that the microglial response is increased after 60 days of treatment when compared to vehicle-treated controls. Since the intracerebral Aβ content of Sideritis spp. treated mice is already reduced after 25 days of treatment, the extracts seem to prolong and/or enhance the beneficial activity of microglia toward plaques. The increased microglial action is aided by an increased expression of ADAM10, primarily reducing Aβproduction but also being the major shedding enzyme for fractalkin (CX3CL1), which plays an important role in microglia-mediated neuroprotection [51]. It has been shown recently that neuron-selective ADAM10 knockout mice show reduced learning ability, synaptic defects, and altered network activity [52]. The increased ADAM10 expression might, therefore, also play a direct role for the enhanced learning abilities of the treated mice. Particularly the extract combination strongly enhances ADAM10 expression, which might explain the improved Morris water maze performance not only in the APP-tg mice. Another very potent mechanism for Aβ clearance is facilitated by the blood-brain barrier transporters, ABCB1 and ABCC1, in vivo [4, 26]. To determine the activating properties of Sideritis spp. extracts on ABCB1 and ABCC1 activity, we used ABC-transporter-activity assays and western blot analyses, but could not find significant effects (data not shown).
To verify whether our in vivo setup and the used mouse model give a realistic estimation of a possible preservation of cognitive abilities in humans, we also treated mice with a Ginkgo biloba extract that is in use in humans [12, 54]. Large patient studies have shown that Ginkgo biloba has no effect on the progression and prevention of AD/cognition. In contrast to the two mouse models that were used for Ginkgo biloba treatment studies so far (TgCRND8 and Tg2576) [55, 56], analyses of our mouse model nicely reflected the finding in patients in showing only minor effects on Aβ42 levels and no effect at all in the Morris water maze performance (Supplementary Figure 1).
We additionally tested the Sideritis spp. extract combination with the focus on cognitive functions in aged, non-tg “healthy” mice in order to assess cognition-enhancing effects. The experimental strategy was a short-term treatment of 15 days just before the Morris water maze tests that were completed at the ages of 150, 300, 450, and 600 days, respectively. Significantly decreased escape latencies were observed for the naïve, wild-type mice that have been treated with the Sideritis spp. extract combination. These results evidence a strong improvement of cognitive performance by means of faster problem solving, storage, and retrieval of memory in young and even in aged mice. Moreover, the animals showed a significantly improved ability to adapt to new information and to retrieve them. At this point, however, it is not clear by which mechanisms this effect was mediated. Further studies are needed to evaluate if cognition enhancement in non-tg mice is also accompanied by lowered mouse Aβ levels and if it (additionally) relies on monoamine reuptake inhibitory properties as shown in rats [17], or other factors like BDNF, neuronal replenishment, or neuronal protection in both non-tg and APP-tg mice. It has been shown that Sideritis scardica exerts antioxidant effects comparable to Camellia sinensis, an effect that might prevent age- and Aβ-related increase of ROS damage by mitochondrial dysfunction [57]. Very recently, a treatment trial in patients has shown that dietary supplementation with Sideritis scardica extract and B-vitamins improves performance in cognitive tasks and executive function especially under stressful conditions [58]. Considering the life threatening approach of the Morris water maze, such properties need to be contemplated.
CONCLUSION
The present study evidences prevention against Aβ-induced memory impairments by herbal extracts of Sideritis scardica and Sideritis euboea in APP-tg mice. These Sideritis species, and in particular the one-to-one combination of both, decreased (i) intracerebral Aβ42, (ii) number and size of amyloid plaques, and (iii) improved memory in APP-tg mice as well as in aged, non-transgenic littermates by enhanced ADAM10 expression and microglia activation. Both Sideritis species are effective at low dosages pre-onset as well as at high dosages after disease onset, without noticeable side effects, neither from this animal study nor from centuries of use in the Balkan region. It will be interesting to elucidate the mechanism of synergism responsible for the enhanced efficacy of the extract combination. Furthermore, a combination of Sideritis spp. with other herbal extracts, known to enhance different AD-relevant mechanisms, like ABC-transporter-activation as seen from low-hyperforin Hypericum perforatum extracts [59, 60], should be elucidated with regard to beneficial synergistic effects in AD pathology. Summarizing, both species of Sideritis bear an auspicious potential for improvement of memory in healthy adults as well as in dementia patients.
ACKNOWLEDGMENTS
We thank Alexandra Sommer, Thomas Brüning, Anne-Sophie Plath, and Gerda Brüsch for their excellent technical support.
The work of J.P. was financed by the following grants: Deutsche Forschungsgemeinschaft DFG PA930/9, DFG PA930/12; VIAA Latvia NFI/R/2014/023; Leibniz Society SAW-2015-IPB-2; HelseSØ No:2016062; Norsk forskningsrådet: NFR251290, NFR246392, NFR248772, JPND NeuroGEM NFR247179, JPND PROP-AD NFR260786.
Authors’ disclosures available online (http://www.j-alz.com/manuscript-disclosures/16-0301r1).
Appendices
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-160301.
REFERENCES
[1] | Dinamarca MC , Arrazola M , Toledo E , Cerpa WF , Hancke J , Inestrosa NC ((2008) ) Release of acetylcholinesterase (AChE) from beta-amyloid plaques assemblies improves the spatial memory impairments in APP-transgenic mice. Chem Biol Interact 175: , 142–149. |
[2] | Klein WL ((2002) ) Abeta toxicity in Alzheimer’s disease: Globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int 41: , 345–352. |
[3] | Cummings JL ((2004) ) Treatment of Alzheimer’s disease: Current and future therapeutic approaches. Rev Neurol Dis 1: , 60–69. |
[4] | Hofrichter J , Krohn M , Schumacher T , Lange C , Feistel B , Walbroel B , Heinze HJ , Crockett S , Sharbel TF , Pahnke J ((2013) ) Reduced Alzheimer’s disease pathology by St. John’s Wort treatment is independent of hyperforin and facilitated by ABCC1 and microglia activation in mice. Curr Alzheimer Res 10: , 1057–1069. |
[5] | Pahnke J , Frohlich C , Paarmann K , Krohn M , Bogdanovic N , Arsland D , Winblad B ((2014) ) Cerebral ABC transporter-common mechanisms may modulate neurodegenerative diseases and depression in elderly subjects. Arch Med Res 45: , 738–743. |
[6] | Lee JW , Lee YK , Ban JO , Ha TY , Yun YP , Han SB , Oh KW , Hong JT ((2009) ) Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J Nutr 139: , 1987–1993. |
[7] | Lee SY , Lee JW , Lee H , Yoo HS , Yun YP , Oh KW , Ha TY , Hong JT ((2005) ) Inhibitory effect of green tea extract on beta-amyloid-induced PC12 cell death by inhibition of the activation of NF-kappaB and ERK/p38 MAP kinase pathway through antioxidant mechanisms. Brain Res Mol Brain Res 140: , 45–54. |
[8] | Rezai-Zadeh K , Arendash GW , Hou H , Fernandez F , Jensen M , Runfeldt M , Shytle RD , Tan J ((2008) ) Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res 1214: , 177–187. |
[9] | Ho L , Chen LH , Wang J , Zhao W , Talcott ST , Ono K , Teplow D , Humala N , Cheng A , Percival SS , Ferruzzi M , Janle E , Dickstein DL , Pasinetti GM ((2009) ) Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J Alzheimers Dis 16: , 59–72. |
[10] | Jayaprakasam B , Padmanabhan K , Nair MG ((2010) ) Withanamides in Withania somnifera fruit protect PC-12 cells from beta-amyloid responsible for Alzheimer’s disease. Phytother Res 24: , 859–863. |
[11] | DeKosky ST , Williamson JD , Fitzpatrick AL , Kronmal RA , Ives DG , Saxton JA , Lopez OL , Burke G , Carlson MC , Fried LP , Kuller LH , Robbins JA , Tracy RP , Woolard NF , Dunn L , Snitz BE , Nahin RL , Furberg CD , Ginkgo Evaluation of Memory (GEM) Study Investigators. ((2008) ) Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 300: , 2253–2262. |
[12] | Vellas B , Coley N , Ousset PJ , Berrut G , Dartigues JF , Dubois B , Grandjean H , Pasquier F , Piette F , Robert P , Touchon J , Garnier P , Mathiex-Fortunet H , Andrieu S , GuidAge Study Group ((2012) ) Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. 11: , Lancet Neurol, 851–859. |
[13] | Musiek ES , Holtzman DM ((2012) ) Origins of Alzheimer’s disease: Reconciling cerebrospinal fluid biomarker and neuropathology data regarding the temporal sequence of amyloid-beta and tau involvement. Curr Opin Neurol 25: , 715–720. |
[14] | Todorova M , Trendafilova A ((2014) ) Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J Ethnopharmacol 152: , 256–265. |
[15] | Gonzalez-Burgos E , Carretero ME , Gomez-Serranillos MP ((2011) ) Sideritis spp.: Uses, chemical composition and pharmacological activities–a review. J Ethnopharmacol 135: , 209–225. |
[16] | Tsaknis J , Lalas S ((2005) ) Extraction and identification of natural antioxidant from Sideritis euboea (mountain tea). J Agric Food Chem 53: , 6375–6381. |
[17] | Knorle R ((2012) ) Extracts of Sideritis scardica as triple monoamine reuptake inhibitors. J Neural Transm 119: , 1477–1482. |
[18] | Tsibranska L , Tylkowski B , Kochanov R , Alipieva K ((2011) ) Extraction of biologically active compounds from Sideritis ssp L. Food Bioproducts Processing 89: , 273–280. |
[19] | Linardaki ZI , Vasilopoulou CG , Constantinou C , Iatrou G , Lamari FN , Margarity M ((2011) ) Differential antioxidant effects of consuming tea from Sideritis clandestina subsp. peloponnesiaca on cerebral regions of adult mice. J Med Food 14: , 1060–1064. |
[20] | Rendeiro C , Guerreiro JD , Williams CM , Spencer JP ((2012) ) Flavonoids as modulators of memory and learning: Molecular interactions resulting in behavioural effects. Proc Nutr Soc 71: , 246–262. |
[21] | Spencer JP ((2010) ) The impact of fruit flavonoids on memory and cognition. Br J Nutr 104: (Suppl 3), S40–S47. |
[22] | Williams RJ , Spencer JP ((2012) ) Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med 52: , 35–45. |
[23] | Kraus B , Wolff H , Heilmann J , Elstner EF ((2007) ) Influence of Hypericum perforatum extract and its single compounds on amyloid-beta mediated toxicity in microglial cells. Life Sci 81: , 884–894. |
[24] | Radde R , Bolmont T , Kaeser SA , Coomaraswamy J , Lindau D , Stoltze L , Calhoun ME , Jaggi F , Wolburg H , Gengler S , Haass C , Ghetti B , Czech C , Holscher C , Mathews PM , Jucker M ((2006) ) Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 7: , 940–946. |
[25] | Krohn M , Bracke A , Avchalumov Y , Schumacher T , Hofrichter J , Paarmann K , Frohlich C , Lange C , Bruning T , von Bohlen Und Halbach O , Pahnke J ((2015) ) Accumulation of murine amyloid-beta mimics early Alzheimer’s disease. Brain 138: , 2370–2382. |
[26] | Krohn M , Lange C , Hofrichter J , Scheffler K , Stenzel J , Steffen J , Schumacher T , Bruning T , Plath AS , Alfen F , Schmidt A , Winter F , Rateitschak K , Wree A , Gsponer J , Walker LC , Pahnke J ((2011) ) Cerebral amyloid-beta proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest 121: , 3924–3931. |
[27] | Scheffler K , Krohn M , Dunkelmann T , Stenzel J , Miroux B , Ibrahim S , von Bohlen Und Halbach O , Heinze HJ , Walker LC , Gsponer JA , Pahnke J ((2012) ) Mitochondrial DNA polymorphisms specifically modify cerebral beta-amyloid proteostasis. Acta Neuropathol 124: , 199–208. |
[28] | Scheffler K , Stenzel J , Krohn M , Lange C , Hofrichter J , Schumacher T , Bruning T , Plath AS , Walker L , Pahnke J ((2011) ) Determination of spatial and temporal distribution of microglia by 230nm-high-resolution, high-throughput automated analysis reveals different amyloid plaque populations in an APP/PS1 mouse model of Alzheimer’s disease. Curr Alzheimer Res 8: , 781–788. |
[29] | Frohlich C , Paarmann K , Steffen J , Stenzel J , Krohn M , Heinze HJ , Pahnke J ((2013) ) Genomic background-related activation of microglia and reduced beta-amyloidosis in a mouse model of Alzheimer’s disease. Eur J Microbiol Immunol (Bp) 3: , 21–27. |
[30] | Morris R ((1984) ) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: , 47–60. |
[31] | Lesne S , Koh MT , Kotilinek L , Kayed R , Glabe CG , Yang A , Gallagher M , Ashe KH ((2006) ) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440: , 352–357. |
[32] | Vasilopoulou CG , Kontogianni VG , Linardaki ZI , Iatrou G , Lamari FN , Nerantzaki AA , Gerothanassis IP , Tzakos AG , Margarity M ((2013) ) Phytochemical composition of“mountain tea” from Sideritis clandestina subsp. clandestina and evaluation of its behavioral and oxidant/antioxidant effects on adult mice. Eur J Nutr 52: , 107–116. |
[33] | Frautschy SA , Yang F , Irrizarry M , Hyman B , Saido TC , Hsiao K , Cole GM ((1998) ) Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol 152: , 307–317. |
[34] | Li C , Zug C , Qu H , Schluesener H , Zhang Z ((2015) ) Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav Brain Res 281: , 32–42. |
[35] | Lesne S , Kotilinek L , Ashe KH ((2008) ) Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience 151: , 745–749. |
[36] | Mattson MP ((2004) ) Pathways towards and away from Alzheimer’s disease. Nature 430: , 631–639. |
[37] | Wang Z , Yang L , Zheng H ((2012) ) Role of APP and Abeta in synaptic physiology. Curr Alzheimer Res 9: , 217–226. |
[38] | Varela-Nallar L , Aranguiz FC , Abbott AC , Slater PG , Inestrosa NC ((2010) ) Adult hippocampal neurogenesis in aging and Alzheimer’s disease. Birth Defects Res C Embryo Today 90: , 284–296. |
[39] | Sperling RA , Laviolette PS , O’Keefe K , O’Brien J , Rentz DM , Pihlajamaki M , Marshall G , Hyman BT , Selkoe DJ , Hedden T , Buckner RL , Becker JA , Johnson KA ((2009) ) Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63: , 178–188. |
[40] | Grutzendler J , Helmin K , Tsai J , Gan WB ((2007) ) Various dendritic abnormalities are associated with fibrillar amyloid deposits in Alzheimer’s disease. Ann N Y Acad Sci 1097: , 30–39. |
[41] | Querfurth HW , LaFerla FM ((2010) ) Alzheimer’s disease. N Engl J Med 362: , 329–344. |
[42] | Naslund J , Haroutunian V , Mohs R , Davis KL , Davies P , Greengard P , Buxbaum JD ((2000) ) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283: , 1571–1577. |
[43] | Lue LF , Kuo YM , Roher AE , Brachova L , Shen Y , Sue L , Beach T , Kurth JH , Rydel RE , Rogers J ((1999) ) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 155: , 853–862. |
[44] | Ma T , Klann E ((2012) ) Amyloid beta: Linking synaptic plasticity failure to memory disruption in Alzheimer’s disease. J Neurochem 120: (Suppl 1), 140–148. |
[45] | Harris JA , Devidze N , Verret L , Ho K , Halabisky B , Thwin MT , Kim D , Hamto P , Lo I , Yu GQ , Palop JJ , Masliah E , Mucke L ((2010) ) Transsynaptic progression of amyloid-beta-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron 68: , 428–441. |
[46] | Commenges D , Scotet V , Renaud S , Jacqmin-Gadda H , Barberger-Gateau P , Dartigues JF ((2000) ) Intake of flavonoids and risk of dementia. Eur J Epidemiol 16: , 357–363. |
[47] | Spencer JP ((2009) ) The impact of flavonoids on memory: Physiological and molecular considerations. Chem Soc Rev 38: , 1152–1161. |
[48] | Letenneur L , Proust-Lima C , Le Gouge A , Dartigues JF , Barberger-Gateau P ((2007) ) Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol 165: , 1364–1371. |
[49] | Qin XY , Cheng Y , Yu LC ((2012) ) Potential protection of green tea polyphenols against intracellular amyloid beta-induced toxicity on primary cultured prefrontal cortical neurons of rats. Neurosci Lett 513: , 170–173. |
[50] | Glass CK , Saijo K , Winner B , Marchetto MC , Gage FH ((2010) ) Mechanisms underlying inflammation in neurodegeneration. Cell 140: , 918–934. |
[51] | Rao JS , Kellom M , Kim HW , Rapoport SI , Reese EA ((2012) ) Neuroinflammation and synaptic loss. Neurochem Res 37: , 903–910. |
[52] | Sastre M , Klockgether T , Heneka MT ((2006) ) Contribution of inflammatory processes to Alzheimer’s disease: Molecular mechanisms. Int J Dev Neurosci 24: , 167–176. |
[53] | Streit WJ ((2006) ) Microglial senescence: Does the brain’s immune system have an expiration date?. Trends Neurosci 29: , 506–510. |
[54] | Snitz BE , O’Meara ES , Carlson MC , Arnold AM , Ives DG , Rapp SR , Saxton J , Lopez OL , Dunn LO , Sink KM , DeKosky ST , Ginkgo Evaluation of Memory (GEM) Study Investigators. ((2009) ) Ginkgo biloba for preventing cognitive decline in older adults: A randomized trial. JAMA 302: , 2663–2670. |
[55] | Liu X , Hao W , Qin Y , Decker Y , Wang X , Burkart M , Schotz K , Menger MD , Fassbender K , Liu Y ((2015) ) Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain Behav Immun 46: , 121–131. |
[56] | Stackman RW , Eckenstein F , Frei B , Kulhanek D , Nowlin J , Quinn JF ((2003) ) Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp Neurol 184: , 510–520. |
[57] | Danesi F , Saha S , Kroon PA , Glibetic M , Konic-Ristic A , D’Antuono LF , Bordoni A ((2013) ) Bioactive-rich Sideritis scardica tea (mountain tea) is as potent as Camellia sinensis tea at inducing cellular antioxidant defences and preventing oxidative stress. J Sci Food Agric 93: , 3558–3564. |
[58] | Behrendt I , Schneider I , Schuchardt JP , Bitterlich N , Hahn A ((2016) ) Effect of an herbal extract of Sideritis scardica and B-vitamins on cognitive performance under stress: A pilot study. Int J Phytomed 8: , 95–103. |
[59] | Pahnke J , Langer O , Krohn M ((2014) ) Alzheimer’s and ABC transporters–new opportunities for diagnostics and treatment. Neurobiol Dis 72 Pt A: , 54–60. |
[60] | Hofrichter J , Krohn M , Schumacher T , Lange C , Feistel B , Walbroel B , Heinze H-J , Crockett S , Sharbel TF , Pahnke J ((2013) ) Reduced Alzheimer’s disease pathology by St. John’s Wort treatment is independent of hyperforin and facilitated by ABCC1 and microglia activation in mice. Curr Alzheimer Res 10: , 1057–1069. |
Figures and Tables
Fig.1
Sideritis spp. extracts improve memory performance in APP-tg mice. A) AD initiation treatment with the Sideritis spp. extract combination improves retentiveness and learning aptitude as shown by the significantly reduced escape latencies compared to vehicle treated APP-tg mice that are similar to vehicle-treated, non-transgenic mice. B) Decreased escape latency values of mice treated post-AD-onset with Sideritis spp. extracts in comparison to vehicle-treated control mice indicate increased spatial memory capabilities (mean + SEM, *p≤0.05).
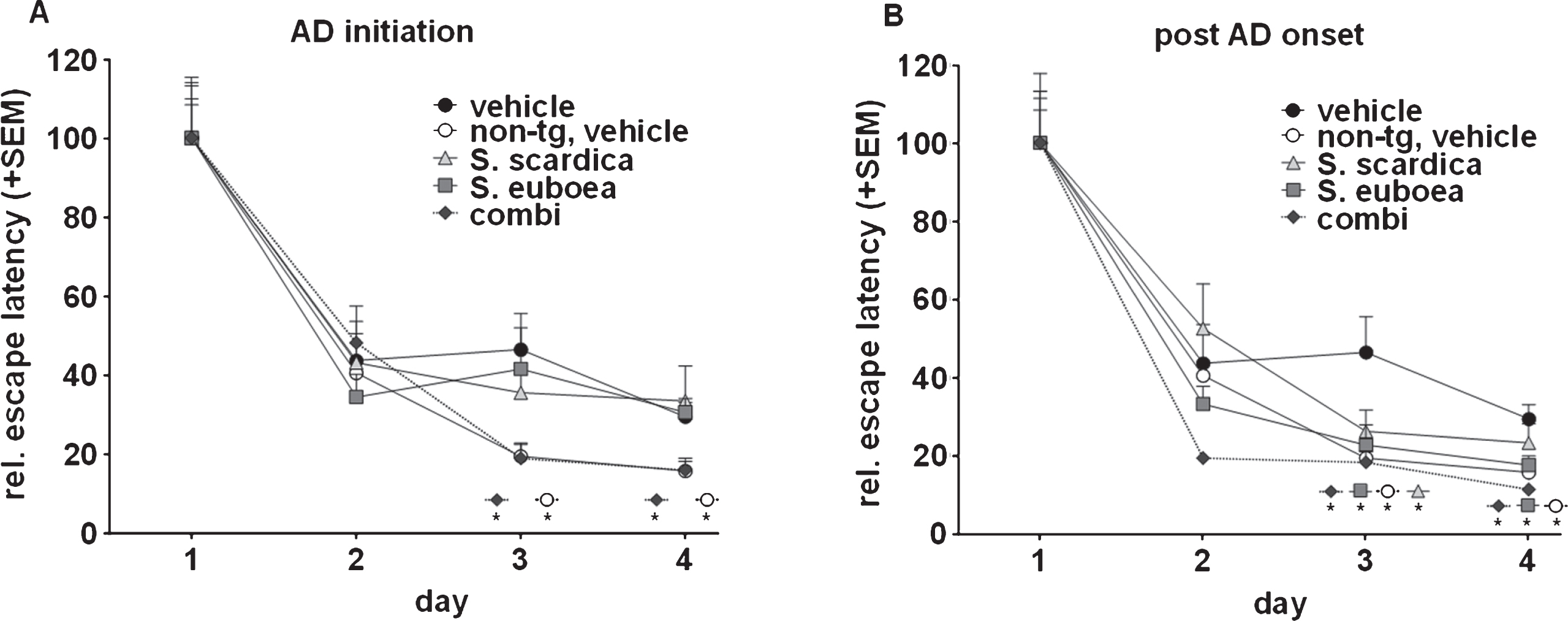
Fig.2
Sideritis spp. potently reduce neurotoxic brain Aβ42 levels and protect from neuronal loss. A) S. scardica, S. euboea, and the extract combination reduce buffer-soluble Aβ42 significantly in both treatment strategies. B) Quantification of neuronal area indicated significant neuron loss in vehicle-treated APP-tg mice in comparison to vehicle-treated, non-transgenic littermates. A significantly increased neuronal area of Sideritis spp.-treated APP-tg mice compared to vehicle treated APP-tg mice indicates neuroprotective effects of the Sideritis spp. extract combination in both paradigms (mean + SEM, *p≤0.05). C, D) Microphotographs of NeuN stained brain slices of APP-tg mice after (C) vehicle treatment and (D) post-AD-onset therapy with Sideritis spp. extract combination (scale bars: 50μm).
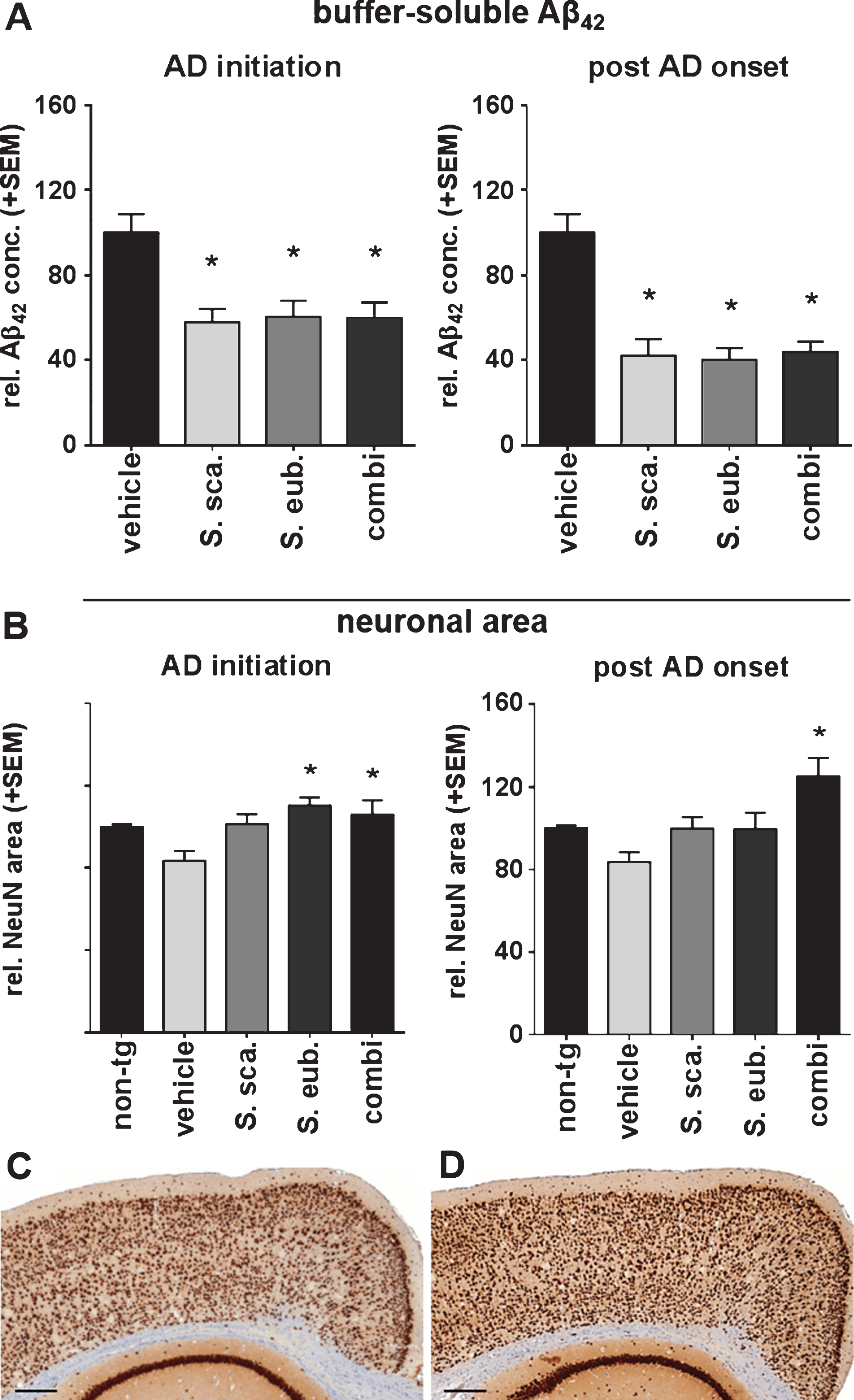
Fig.3
Sideritis spp. extracts restrict Aβ plaque deposition and growth in APP-tg mice. A-D) Exemplary microphotographs of cortical sections stained against Aβ after (A) vehicle, (B) S. scardica, (C) S. euboea, and (D) extract combination in the post-AD-onset treatment (scale bars: upper row 500μm, lower row 50μm). E, F) Sideritis spp. extract combination reduced plaque size when applied according to AD-initiation treatment strategy. G, H) post-AD-onset treatment significantly reduced both, plaque number and size in all extract groups versus controls (mean + SEM, *p≤0.05).
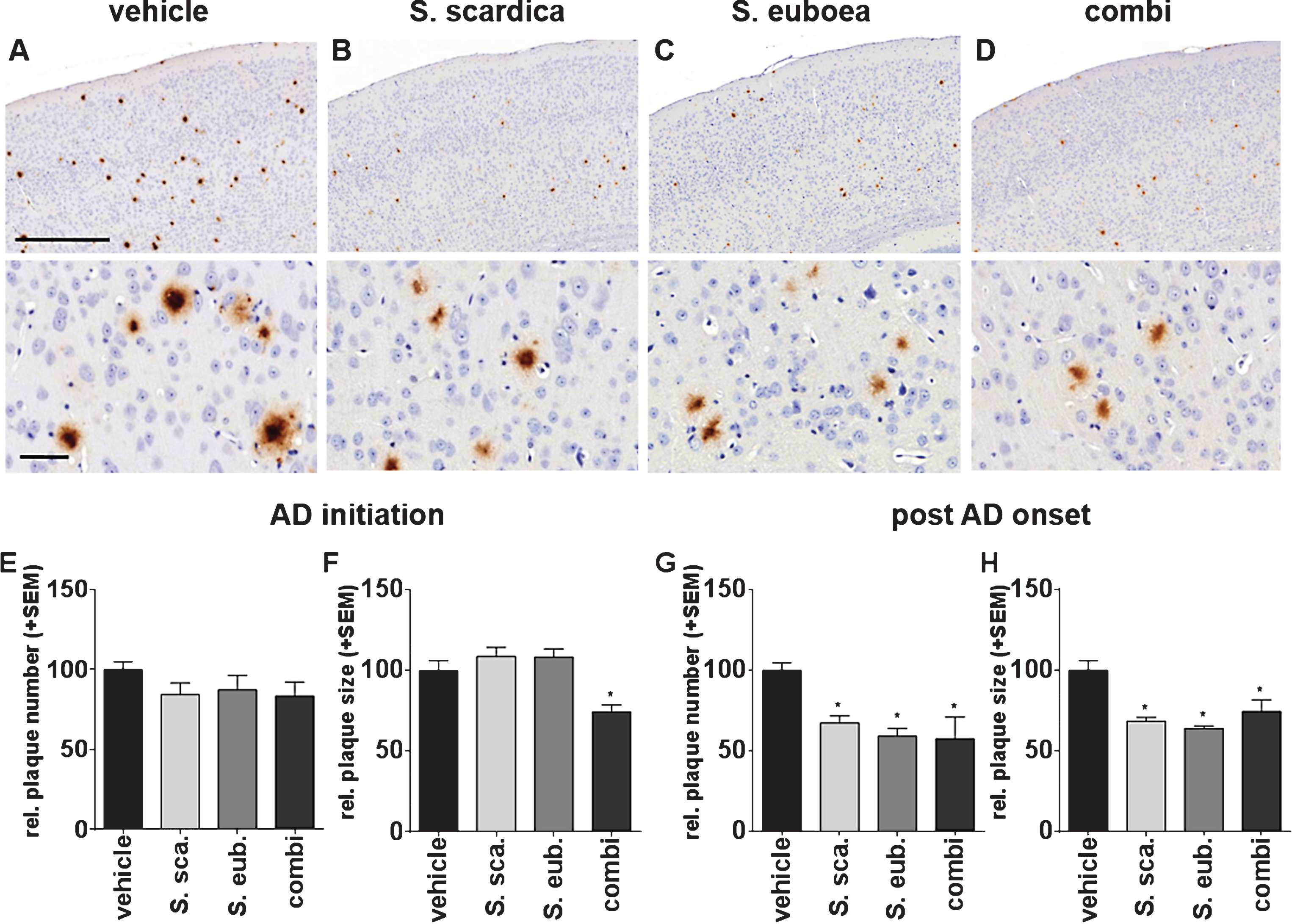
Fig.4
Sideritis spp. extracts enhance microglia activity. A-D) Representative microphotographs of cortical regions of mice treated with Sideritis spp. extracts, co-stained for microglia (brown) and Aβ (red): (A) vehicle, (B) Sideritis scardica, (C) Sideritis euboea, and (D) the extract combination (scale bars: Upper row 500μm, lower row 50μm). E-H) For quantification of the microglia area, only microglia in the vicinity of plaques were included. Microglia area was significantly increased after (E) AD-initiation and (G) post-AD-onset treatment paradigms, while the relative number of plaques covered to at least 50% by microglia was unchanged in (F, H) both paradigms (mean + SEM, *p≤0.05).
Fig.5
Sideritis spp. extracts increase ADAM10 expression. A, B) Expression levels of the alpha-secretase were shown by western blot analyses of brain homogenates of APP-tg mice. C, D) Both AD-initiation and post-AD-onset extract application significantly increased ADAM10 expression in all extract groups. E, F) A significant change in the BACE1 expression was only observed during AD-initiation treatment (mean + SEM, *p≤0.05).
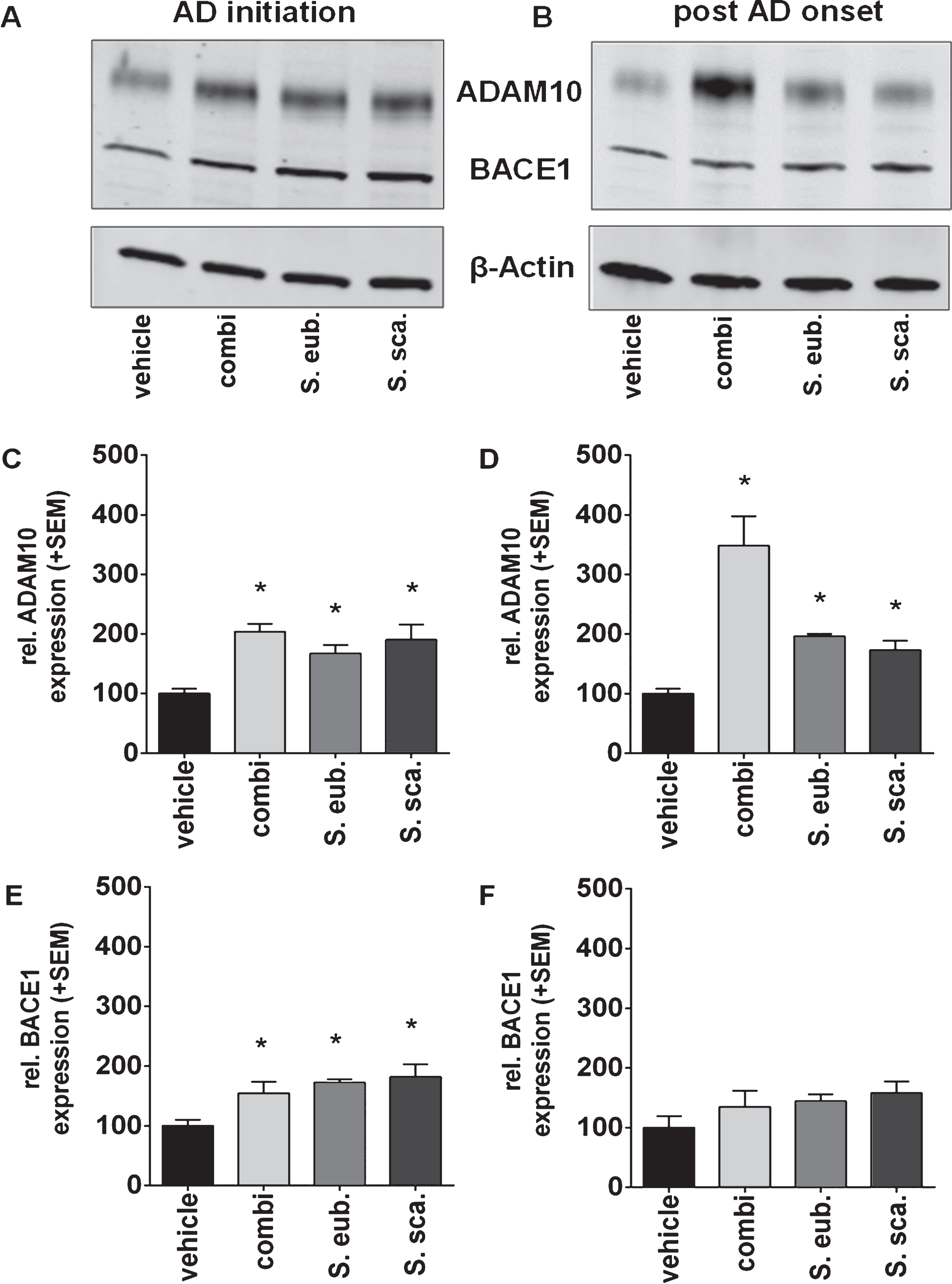
Fig.6
Sideritis spp. treatment stabilizes Aβ42 levels. Calculation of the fold-increase of buffer-soluble Aβ42 levels reveals that post-AD-onset treatment with Sideritis spp. extracts cuts the increase from the age of 50 d (treatment start) to 100 d by at least half and thus stabilizes the amount of Aβ42 at the level of 75-days-old animals (represented by the dotted line). *indicates significant difference to vehicle; #indicates significant difference to 75-days-old, untreated mice (i.e., dotted line at 1.0) (mean + SEM, *p < 0.05).
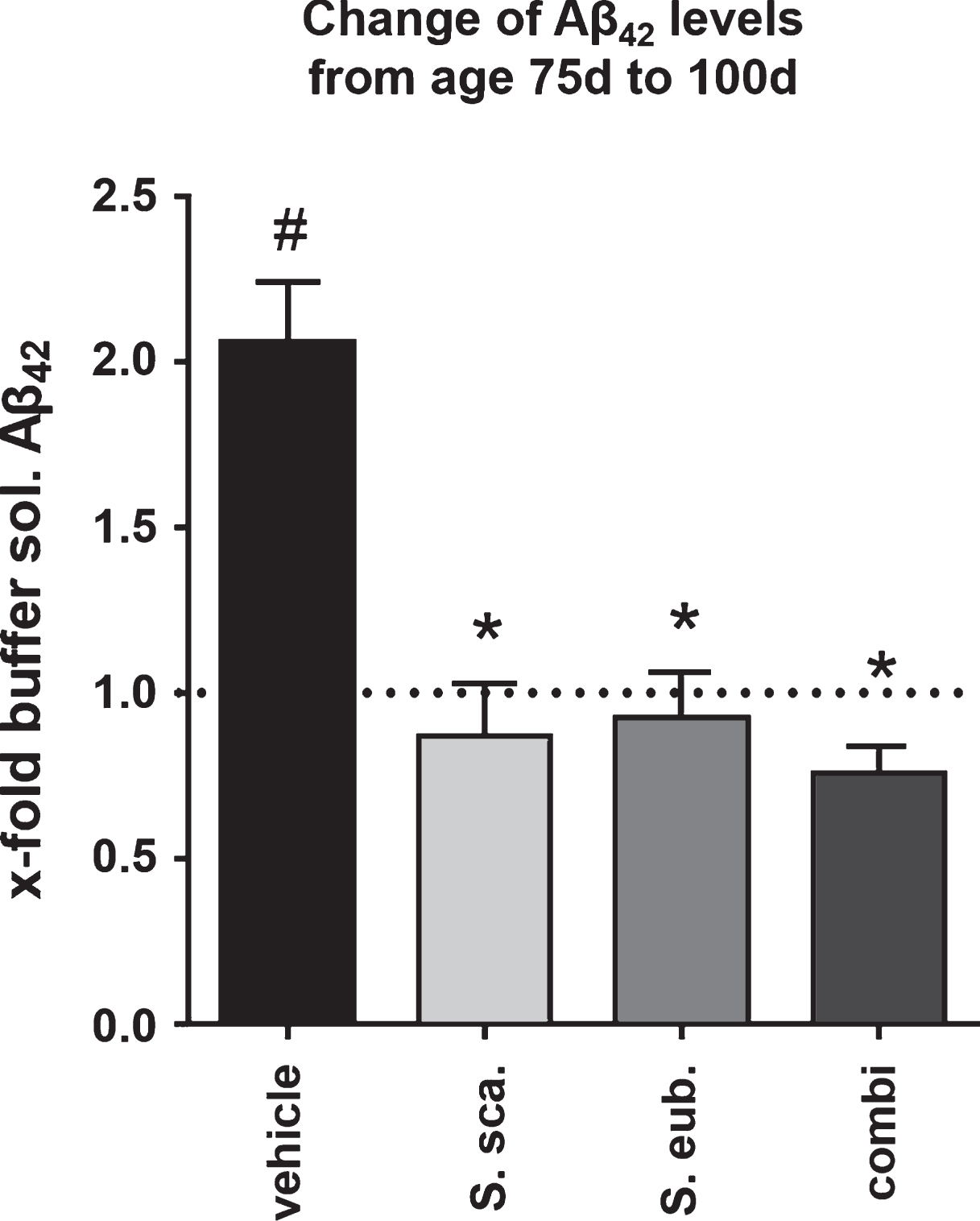
Fig.7
The Sideritis spp. extract combination improved cognition in young and old, non-transgenic ‘healthy’ mice. Reduced water maze escape latency values of treated mice at an age of 150 days as well as at later time points reveal enhanced cognition. Comparison of the escape latency of the second test day with the escape latency of the first day’s second trial indicate an enhanced ability to adapt to new information at all tested time points (mean + SEM, *p≤0.05).
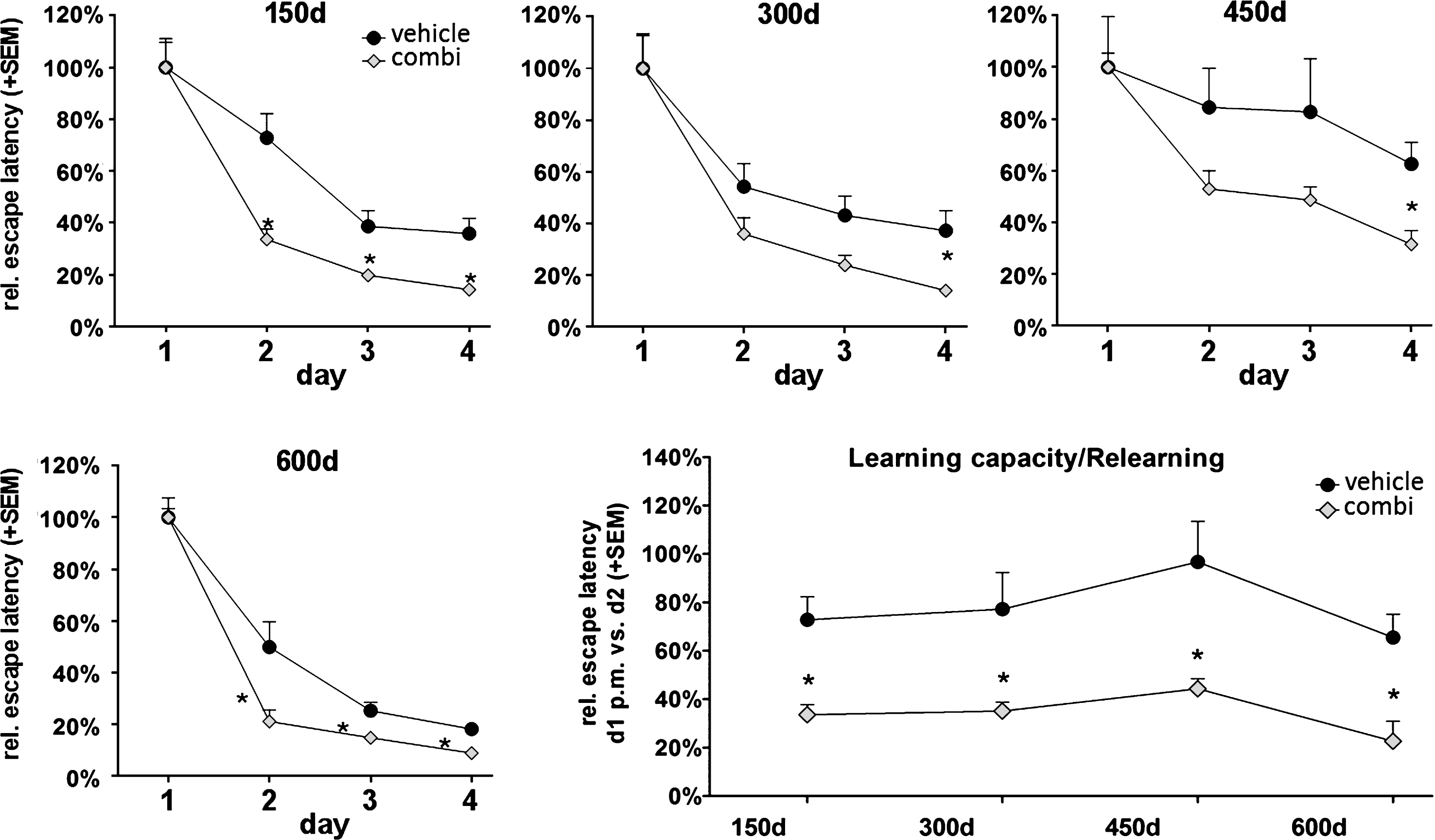
Table 1
Sideritis spp. extracts used for the treatment of APP-tg and non-tg mice: Sideritis species, solvents, drug-extract-ratio (DER), and animal number used for investigations
| Group | Extraction solvent (V/V) | DER native | Animals (n) AD-initiation / post- AD-onset | |
| APP-tg | Sideritis euboea | 20% ethanol | 6:1 | 6 / 6 |
| Sideritis scardica | 20% ethanol | 6:1 | 6 / 5 | |
| Sideritis extract combination | 20% ethanol | 6:1 | 6 / 10 | |
| vehicle (controls) | – | – | 9 | |
| non-tg | Sideritis extract combination | 20% ethanol | 6:1 | 6 |
| vehicle (controls) | – | – | 7 |



