Lewy Bodies, Vascular Risk Factors, and Subcortical Arteriosclerotic Leukoencephalopathy, but not Alzheimer Pathology, are Associated with Development of Psychosis in Alzheimer’s Disease
Abstract
Background:
The neuropathological correlates of psychosis in Alzheimer’s disease (AD) is unclear, with some studies reporting a correlation between psychosis and increased AD pathology while others have found no association.
Objective:
To determine the demographic, clinical, and neuropathological features associated with psychotic symptoms in clinically attributed and neuropathologically proven AD.
Method:
We separately reviewed two overlapping groups of clinically diagnosed (cAD) AD patients with neuropathology data and neuropathologically definite (npAD) cases (regardless of clinical diagnosis) from the NACC database, and explored the relationships between psychosis and clinical variables, neuropathologic correlates, and vascular risk factors. Delusions and hallucinations, defined according to the NPI-Q, were analyzed separately.
Results:
1,073 subjects in the database fulfilled our criteria (890 cAD and 728 npAD patients). 34% of cAD and 37% of npAD had psychotic symptoms during their illness. Hallucinations were associated with greater cognitive and functional impairments on the MMSE and CDR, while delusional patients showed less impairment on CDR, consistent across cAD and npAD groups. Burden of AD pathology appears to relate to presence of psychotic symptoms in the clinical AD group, but this result is not confirmed in the neuropathologically confirmed group suggesting the findings in the clinical group were due to misdiagnosis of AD. Lewy body pathology, subcortical arteriosclerotic leukoencephalopathy, and vascular risk factors, including a history of hypertension and diabetes, were associated with the development of psychosis.
Method:
Vascular and Lewy body pathologies and vascular risk factors are important modifiers of the risk of psychosis in AD.
INTRODUCTION
It is estimated that approximately 50% of patients with Alzheimer’s disease (AD) will develop symptoms of psychosis (delusions and/or hallucinations) at some point in the course of the disease process [1]. Frequencies of these symptoms may vary, with approximately 19% of community dwelling AD patients thought to have delusions and approximately 14% thought to have hallucinations [2]. While delusions are more common than hallucinations, they are often associated with greater insight and therefore are less problematic [3]. Psychotic symptoms in AD are clinically significant, as they have been shown to be associated with increased caregiver burden [4], increased functional decline [4], and more rapid disease progression [5]. The pathobiological mechanisms that underlie psychotic symptoms are unclear, limiting our ability to manage and treat these symptoms.
Previous studies examining clustering of neuropsychiatric symptoms in AD have consistently demonstrated psychosis to be a reliable and valid symptom cluster [6–9]. In fact, Canevelli et al. [10]examined numerous behavioral and psychological sub syndromes using the Neuropsychiatric Inventory Questionnaire (NPI-Q) and found psychosis to be the only reliable and valid symptom cluster. Challenges relating to disentangling these symptoms, both clinically and neurobiologically, are that patients may have either delusions, hallucinations, or both, and it is possible that the different symptoms themselves may have different neurobiological correlates [11]. Most studies to date examining the neurobiological correlates of psychosis in AD have failed to separate delusions from hallucinations, thus limiting their conclusions.
Neuropathological studies investigating psychosis in AD have produced conflicting results, with some studies showing a correlation with severity of Alzheimer pathology [12–14], including neuritic plaques (NPs) and neurofibrillary tangles (NFTs), and others showing no such association [15, 16]. Sweet and colleagues [15] compared the brains of 24 AD patients with psychosis to 25 patients without psychosis, controlling for Lewy bodies (LBs), and found no correlation with NPs or NFTs, thus suggesting that psychotic symptoms in AD may be mediated by an alternate pathway. In contrast, Farber and colleagues [12] followed 109 AD subjects until death and found that the 63% of the sample that developed psychotic symptoms over their disease course had a 2.3 times higher NFT load when compared to patients without psychosis, independent of dementia severity. Murray and colleagues [14] compared AD patients with and without psychosis and found significantly higher rates of phosphorylated tau in the prefrontal cortex of the psychotic group. Meanwhile, Koppel and colleagues [17] found significantly higher levels of phosphorylated tau in the frontal cortex only in females AD patients, while psychosis in males was associated with the presence of α-synuclein pathology, suggesting a possible gender-related effect.
The disparities also appear in relation to cerebrospinal fluid (CSF). Skogseth and colleagues [18] examined CSF of 32 AD patients with neuropsychiatric symptoms and found no correlation between psychosis and tau or amyloid-β levels. However, Koppel and colleagues [13] compared CSF samples of AD patients with and without psychosis and found a correlation with tau levels. In a different disease group, Ballard and colleagues [16] examined 112 autopsy cases of patients with dementia with Lewy bodies (DLB) and found an inverse relationship between NFT burden and psychosis, suggesting that symptoms in this sample were not associated with increased Alzheimer pathology.
Imaging studies examining the correlates of psychosis in AD to date have produced mixed results. Ismail and colleagues [19] in a recent systematic review pooled imaging studies looking at delusions in AD and found an association with right frontal atrophy. The vast majority of imaging studies looking at psychosis in AD cross-sectionally have shown grey matter volume loss most prominent in the frontal lobes [20]. Rosenberg et al. [21] conducted a recent review of brain circuits associated with neuropsychiatric symptoms in AD and concluded that delusions are associated with abnormalities in the frontal cortex, including orbito-frontal, dorsolateral prefrontal, and inferior frontal. Additional brain structures include the right and left insula, and studies have been inconsistent with regard to lateralization. Our group also looked at this question with respect to delusions and found cross-sectionally grey matter volume loss increased in the right-frontal-temporal region [22]. We also examined patients longitudinally and found atrophy more pronounced in regions corresponding to the default mode network and cerebellum [23]. As none of the regions identified to date correspond to areas of atrophy in AD, combined they suggest a different mechanism may underlie the development of delusions in AD.
The association of psychosis with cerebrovascular risk factors is a recent topic of interest. Steinberg et al. [24] examined the correlation between cardiovascular risk factors and neuropsychiatric symptoms in AD patients, finding a correlation only with antihypertensive medications. There have been numerous studies suggesting white matter hyperintensities may play a role in depression and mild cognitive impairment as well as early AD [25]. However, few studies have examined postmortem markers of cerebrovascular disease in AD patients with psychosis.
Research to date has yet to reach a conclusion regarding the neuropathological correlates of psychosis in AD, although it is possible based on prior studies that Alzheimer pathology, cerebrovascular disease and LBs may all play a role. Limitations of previous studies include the fact that very few studies distinguished delusions from hallucinations; the sample sizes were small in many cases; most studies excluded analyses of vascular pathology; and correlations were made to clinical diagnosis without neuropathological confirmation. We plan to explore the role of Alzheimer pathology, LBs and cerebrovascular disease in the development of psychotic symptoms in AD using data from the National Alzheimer’s Coordinating Center (NACC) database, and to compare our findings in clinically diagnosed AD and neuropathologically confirmed patients as well as sub-stratify patients into delusions versus hallucinations. We predict that our findings will differ across the subgroups, reflecting different disease mechanisms for each symptom.
METHODS
Data source
Data were obtained from the NACC Uniform Data Set (UDS) and Neuropathology (NP) Data Set collected between September 2005 and May 2012 [26]. This analysis used data from 29 Alzheimer’s Disease Centers. The following data were analyzed from the UDS: demographic data including gender and years of education; disease duration calculated from the age of cognitive decline (not age of dementia diagnosis) to age of death, based on a clinician’s assessment; vascular risk factors including a history of hypertension, diabetes, hypercholesterolemia, and smoking; Functional Activity Questionnaire (FAQ) as a measure of Activities of Daily Living [27]; and cognitive performance on the Mini-Mental State Exam (MMSE) [28] and the global Clinical Dementia Rating (CDR) [29]. The presence of delusions and/or hallucinations was identified by the NPI-Q, which measures 12 categories of behavioral disturbances. The questionnaire is completed by a caregiver and asks about the severity and caregiver distress level for each category.
The NP data include the Braak & Braak stage for NFTs (Stages I-VI) [30], the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) neuropathological criteria (Definite, Probable, or Possible AD; [31]), NIA/Reagan Institute neuropathological criteria (high/intermediate/low likelihood of dementia being AD [32]), a count of NPs based on the most severely affected cortical region [33], the criteria modified from McKeith et al. for DLB [34], and an assessment of vascular pathology. Vascular pathology evaluation included the presence or absence of any gross or microscopic vascular pathology, including lacunes (cystic/old infarcts or hemorrhages ≤1 cm in diameter), cortical microinfarcts, hemorrhages, hippocampal sclerosis, and subcortical arteriosclerotic leukoencephalopathy (SAL— multifocal or diffuse white matter pathology attributable to arteriosclerotic small vessel disease [35, 36]).
All participants had standardized clinical evaluations and received follow-ups approximately annually for as long as the subject was able and willing to participate. The current study was approved by the St. Michael’s Hospital Research Ethics Board.
Eligibility criteria
Patients with a primary clinical diagnosis of probable AD (cAD) based on the NINCDS-ADRDA criteria [37] with available pathologic data and patients with a neuropathological diagnosis of AD (npAD) as defined by “Definite AD” on the CERAD [31] were included in the analysis. Brain injury, CNS neoplasm, Down syndrome, Huntington’s disease, and prion disease were exclusion factors.
The presence of delusions or hallucinations was identified by a score of 1 on the NPI-Q [38] delusional and hallucinatory items, respectively, at any of the visits. As the persistence of psychosis in AD is low and rarely persists after a few months [1], patients with psychotic symptoms at any visit were included in the psychotic group. Clinically diagnosed AD subjects with neither item endorsed at any visits were considered never psychotic (cAD–P) while subjects who had psychosis at any visit were considered psychotic (cAD+P). The cAD+P group was further classified into i) delusional (only the presence of delusions at any visit; cAD+D), ii) hallucinatory (only the presence of hallucinations; cAD+H), or iii) duo-psychotic (both delusions and hallucinations at any visit; cAD+DH). Neuropathologically-confirmed AD subjects without any psychosis were designated as npAD-P while those with any history of psychosis were designated as npAD+P. Similarly, npAD+P patients were further classified into npAD+D for delusions, npAD+H for hallucinations, or npAD+DH for duo-psychosis.
Statistical analysis
cAD+P and its subgroups (cAD+D, cAD+H, and cAD+DH) were individually compared to cAD–P using univariate tests. The χ2 test of independence was used for categorical data, ordinal logistic regression was used for ordinal data, and the Mann-Whitney test was used for continuous data. With respect to the latter, a non-parametric test was adopted instead of the parametric t-test because Kolmogorov-Smirnov test of normality was statistically significant. Similarly npAD+P, npAD+D, npAD+H, and npAD+H groups were individually compared to npAD-P subjects. All statistical analysis was performed using SPSS 19.0 and statistical significance was assessed using α= 0.05.
RESULTS
We identified 1,073 subjects in the NACC database who fulfilled the criteria for analysis with available neuropathology data, including 890 cAD and 728 npAD subjects (Fig. 1). The cAD and npAD groups are overlapping groups as not all the cAD subjects had a neuropathological diagnosis of AD. Of the npAD patients who were not clinically diagnosed with AD (false negatives, n = 116), 42 (36.2%) were diagnosed with DLB, 11 (9.5%) were diagnosed with Parkinson’s disease dementia, 12 (10.3%) were diagnosed with vascular dementia, and 51 (44.0%) were diagnosed with possible AD. The average MMSE score of the npAD group was 13.6±8.0, so the group appeared to meet the threshold for dementia.
Demographic and clinical variables
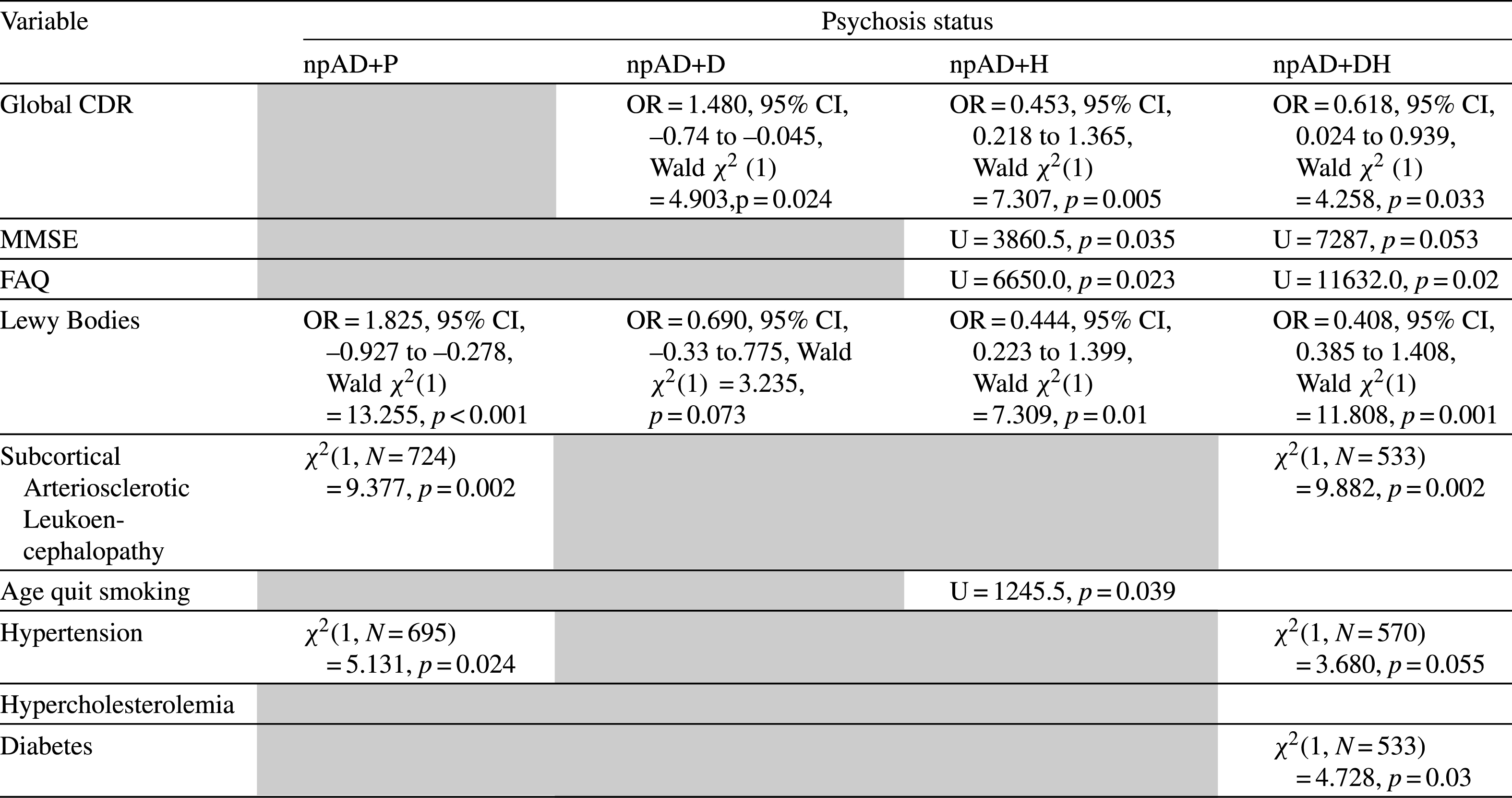
There were no statistically significant differences between cAD–P (Tables 1 and 3) and its psychotic groups as well as between npAD-P (Tables 2 and 4) and its psychotic groups with respect to age of death, years of education, ethnicity, and gender. We looked at the relationship of psychotic symptoms to FAQ, MMSE, and CDR on the last visit prior to death. AD+P groups did not differ from AD-P in both cAD and npAD cohorts on the MMSE, CDR, or FAQ but further breakdown of the psychotic groups showed that AD+H and AD+DH were associated with more cognitive impairment on the MMSE and CDR, as well as greater functional impairment on the FAQ compared to AD-P, although the npAD+DH group did not reach significance on the MMSE. On the contrary, AD+D cohorts were associated with less impairment than AD-P on global CDR. However, cAD+P, cAD+D, cAD+DH, and npAD+D patients had a significantly longer duration from last clinical visit to death than their respective non-psychotic control groups. In addition, cAD+P, cAD+D, and cAD+DH groups had longer disease durations compared to cAD–P.
Neuropathology analyses
Autopsy findings revealed that, compared to cAD–P, there was significantly higher Braak staging in cAD+P, cAD+D, and cAD+DH, more frequent neuritic plaques in cAD+P and cAD+D, as well as greater AD burden on the NIA Reagan and on the CERAD in all psychotic cAD groups. In contrast, in the neuropathologically confirmed AD group, there were no differences in Braak stage, plaque count, or NIA Reagan between npAD-P and any of the psychotic subgroups. We found significantly more LB pathology in cAD+P and cAD+D groups compared to cAD–P, and similarly in npAD+P, npAD+H, and npAD+DH groups compared to npAD–P. There were a greater proportion of patients who had SAL in AD+P and AD+DH groups, consistent across both clinical and neuropathological AD cohorts compared to AD-P groups. There was no statistically significant association of psychosis with gross or microscopic vascular pathology, presence of microinfarcts, lacunes, hippocampal sclerosis, or hemorrhages in either cAD or npAD cohorts.
Vascular risk factor analyses
A history of hypertension was associated with cAD+P, cAD+DH, and npAD+P groups compared to their non-psychotic counterparts. A history of diabetes was associated with AD+DH in both cAD and npAD cohorts. Lastly, AD+H subjects had an increased odds of smoking more packs of cigarette per day in the cAD group as well as quit smoking at a later age in both cAD and npAD groups compared to AD-P. The results were replicated using the NIA-Reagan neuropathological criteria, and results can be found in Supplementary Tables 1 and 2.
DISCUSSION
Our samples of cAD and npAD patients with and without psychosis were matched demographically with regards to age and education. Concordant clinical findings observed in both cAD and npAD samples included the fact that AD+P patients were not more functionally impaired at their last clinical visit compared to the AD-P group, contrary to existing literature that suggests psychosis represents a more severe form of AD [1, 5, 6, 39]. However, in breaking down symptoms into delusions and hallucinations, we found a clear separation in terms of impact on disease severity. AD patients with hallucinations, both with and without delusions, had more advanced disease (higher CDR scores) and were more cognitively and functionally impaired (lower MMSE scores and higher FAQs) at the last study visit prior to death, although in the latter there was no association observed between hallucinations and cognition in the npAD group. Contrary to previous studies [4], AD+D subjects were not more functionally impaired and, in fact, had significantly less clinical disease severity at the visit prior to death when compared to AD–P subjects as measured by the MMSE and global CDR. One potential explanation for this finding is that patients with delusions had a longer interval between the last clinical assessments to time of death, so it is possible that the disease progression was less advanced at the last assessment. Another explanation for this finding is that a certain level of cognitive ability is required to form and express a delusion, and that these symptoms remit as cognition declines.
In terms of discordant clinical findings between the cAD and npAD samples, patients in the cAD sample with psychosis had a longer duration of illness. This could indicate slower progression and thus less disease severity in patients with AD and psychosis. This would be contrary to some existing literature, which suggests that patients with psychosis undergo a more rapid disease progression [5]. One potential explanation is that patients with psychosis, due to the nature of their symptoms, may seek medical attention earlier, thus creating an artificially longer disease duration. An alternative explanation is that perhaps the rate of decline varies at different stages of the AD continuum, with more rapid progression in earlier stages. Currently, the literature is equivocal in regards to associations of AD+P and age of onset and illness duration [1].
In terms of the neuropathological analyses, concordant findings between the cAD and npAD sample included the observation that LBs were correlated with psychosis. The cAD sample showed a stronger correlation with delusions while the npAD sample showed a stronger association with hallucinations. The finding of association of psychosis with LB is consistent with Jacobson et al. [40], who found a strong correlation between visual hallucinations and LBs, both in patients with DLB and clinical AD, and a rather poor correlation with other markers of AD neuropathology (NFTs and NPs). These findings are also consistent with the work of Ballard et al. [16] who concluded that hallucinations in patients with DLB are likely mediated by an alternate disease mechanism from AD pathology, as he observed them to be inversely related to both NPs and NFTs.
As well, in terms of vascular pathology, psychosis was not correlated with specific lesions such as stroke and lacunar infarcts, but both cohorts showed a correlation between AD+P/AD+DH and SAL. Hypertension (in AD+P), diabetes (in AD+DH) and age at quitting smoking (AD+H) were also positively correlated with psychotic symptoms. Overall, all the vascular risk factors appeared to be positively associated with psychosis and its subgroups, although many of them did not reach significance. This provides the first compelling evidence that psychotic symptoms in AD patients may be mediated by a vascular mechanism. This is somewhat consistent with the findings of Steinberg et al. [24] who correlated NPI-Q scores, specifically the affective cluster, with antihypertensive use but found no other associations. Bidzan et al. [41] similarly found that vascular factors, as measured by the Hachinski score, were correlated with depression and anxiety, but not with hallucinations or delusions. Unfortunately we did not look at anxiety and depressive symptoms in our study so it is hard to comment on how strong this correlation is in relation to affective symptoms. Previous research found that vascular burden may disrupt local connections between the frontal lobe and subcortical areas through disruptions of cholinergic transmissions, which may intensify neuropsychiatric symptoms [42]. Our findings suggest that targeting the modifiable aspects, such as managing vascular risk factors (e.g., controlling diabetes and hypertension), may reduce the risk of psychosis.
We found a number of significant discordant neuropathological findings when comparing the cAD and npAD groups. In cAD patients, psychosis was associated with more advanced Alzheimer pathology, including Braak stage, NIA Reagan scores, CERAD scores, and NPs. Specifically, advanced Braak stage was associated with delusions with or without hallucinations; NIA Reagan scores correlated with all psychotic groups; CERAD scores correlated with both delusions and hallucinations; and NPs correlated with delusions only. These findings are consistent with prior studies [12, 14] and support the concept that AD+P may represent a more aggressive form of the disease, associated with more advanced neuropathology [5]. Importantly, however, there were no significant correlations between Alzheimer pathology and psychotic symptoms in patients with npAD, suggesting that the observed findings in cAD may be driven by misdiagnosis, specifically false positive diagnosis of AD. While it would be of interest to know what the ultimate diagnosis was in the case of patients who were false positive, unfortunately this data was not available. Our paper is the first to highlight the differences between cAD and npAD with regard to psychosis and Alzheimer pathology and may well explain the discrepancy in the field to date. The majority of studies reporting a positive [12–14] and negative [15, 18] correlation with Alzheimer pathology relied on clinical diagnosis as opposed to neuropathologically confirmed diagnosis. However, clinical and neuropathological expression of AD is not always correlated. Monsell et al. found that some patients who have AD neuropathology are asymptomatic despite neuritic plaque burden, and suggested that neurofibrillary tangles has a larger role in determining the clinical expression of AD [43]. Another potential explanation for the discrepancy between cAD and npAD may reflect differences in disease severity. In order to meet criteria for npAD, patients by definition must have severe disease while patients with cAD may have had a broader range of disease severity, thus making it easier for an association to be detected.
There are some limitations of the current study. We relied on data obtained from different centers across the United States, so it is possible that there was some variation in the collection of data. We also used many univariate tests in our statistical analysis, which increased the probability of a Type I error. Moreover, we used clinical data obtained from the last study visit, whether or not the patients had active psychotic symptoms at that time, as opposed to looking at the course of symptoms over time. There was also variation in the time between last visit and time of death, which may have affected the results. Additional, prospective studies will be needed to better define different trajectories of disease in patients with and without psychosis. It is possible that some patients with active psychotic symptoms in fact had delirium, given it may be hard to differentiate the two conditions in advanced dementia. The NPI-Q captures symptoms that have occurred in the previous month, so it is possible that some informant reports may reflect a delirium that had resolved by the time of the study visit. Also, it is possible that clinical differences between patients were not detected secondary to the floor effects of the instruments used (MMSE, CDR, FAQ) given that most patients had advanced dementia.
In summary, AD patients with psychotic symptoms do not appear to differ demographically or clinically from AD patients without psychosis, but further breakdown of psychotic symptoms showed that patients with hallucinations appear more cognitively and functionally impaired at last visit prior to death, while there is some evidence to suggest that delusions are associated with better cognitive and functional status at last visit prior to death. We demonstrated that in the clinically diagnosed AD population, including false positive diagnosis of AD, psychosis is associated with AD pathology. However, in the pathologically diagnosed AD population, psychotic symptoms do not vary with the severity of AD pathology but instead are associated with LBs, subcortical ischemic vasculopathy, and cerebrovascular risk factors including hypertension, smoking, and diabetes. Combined, these findings suggest that LBs and cerebrovascular disease are important risk modifiers of psychosisin AD.
ACKNOWLEDGMENTS
This study is funded by the Canadian Institutes of Health Research (CIHR) [Grant number 313912].
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI David Teplow, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), and P50 AG005681 (PI John Morris, MD)
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0606r2).
Appendices
The supplementary table and figure are available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150606.
REFERENCES
[1] | Ropacki SA , Jeste DV ((2005) ) Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990 to 2003. Am J Psychiatry 162: , 2022–2030. |
[2] | Lyketsos CG , Steinberg M , Tschanz JT , Norton MC , Steffens DC , Breitner JC ((2000) ) Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. Am J Psychiatry 157: , 708–714. |
[3] | Ballard CG , Bannister CL , Patel A , Graham C , Oyebode F , Wilcock G , Chung MC ((1995) ) Classification of psychotic symptoms in dementia sufferers. Acta Psychiatr Scand 92: , 63–68. |
[4] | Fischer CE , Ismail Z , Schweizer TA ((2012) ) Delusions increase functional impairment in Alzheimer’s disease. Dement Geriatr Cogn Disord 33: , 393–399. |
[5] | Sweet RA , Bennett DA , Graff-Radford NR , Mayeux R ((2010) ) Assessment and familial aggregation of psychosis in Alzheimer’s disease from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Brain 133: , 1155–1162. |
[6] | Hollingworth P , Hamshere ML , Moskvina V , Dowzell K , Moore PJ , Foy C , Archer N , Lynch A , Lovestone S , Brayne C , Rubinsztein DC , Lawlor B , Gill M , Owen MJ , Williams J ((2006) ) Four components describe behavioral symptoms in 1,120 individuals with late-onset Alzheimer’s disease. J Am Geriatr Soc 54: , 1348–1354. |
[7] | Kang HS , Ahn IS , Kim JH , Kim DK ((2010) ) Neuropsychiatric symptoms in korean patients with Alzheimer’s disease: Exploratory factor analysis and confirmatory factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn Disord 29: , 82–87. |
[8] | Cheng ST , Kwok T , Lam LC ((2012) ) Neuropsychiatric symptom clusters of Alzheimer’s disease in Hong Kong Chinese: Prevalence and confirmatory factor analysis of the Neuropsychiatric Inventory. Int Psychogeriatr 24: , 1465–1473. |
[9] | Bettney L , Butt S , Morris J , Connolly A , McCollum C , Burns A , Purandare N ((2012) ) Investigating the stability of neuropsychiatric sub-syndromes with progression of dementia: A 2-year prospective study. Int J Geriatr Psychiatry 27: , 1118–1123. |
[10] | Canevelli M , Adali N , Voisin T , Soto ME , Bruno G , Cesari M , Vellas B ((2013) ) Behavioral and psychological subsyndromes in Alzheimer’s disease using the Neuropsychiatric Inventory. Int J Geriatr Psychiatry 28: , 795–803. |
[11] | Ismail Z , Nguyen MQ , Fischer CE , Schweizer TA , Mulsant BH , Mamo D ((2011) ) Neurobiology of delusions in Alzheimer’s disease. Curr Psychiatry Rep 13: , 211–218. |
[12] | Farber NB , Rubin EH , Newcomer JW , Kinscherf DA , Miller JP , Morris JC , Olney JW , McKeel DW Jr ((2000) ) Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch Gen Psychiatry 57: , 1165–1173. |
[13] | Koppel J , Sunday S , Buthorn J , Goldberg T , Davies P , Greenwald B , Alzheimer’s Disease Neuroimaging Initiative ((2013) ) Elevated CSF tau is associated with psychosis in Alzheimer’s disease. Am J Psychiatry 170: , 1212–1213. |
[14] | Murray PS , Kirkwood CM , Gray MC , Fish KN , Ikonomovic MD , Hamilton RL , Kofler JK , Klunk WE , Lopez OL , Sweet RA ((2014) ) Hyperphosphorylated tau is elevated in Alzheimer’s disease with psychosis. J Alzheimers Dis 39: , 759–773. |
[15] | Sweet RA , Hamilton RL , Lopez OL , Klunk WE , Wisniewski SR , Kaufer DI , Healy MT , DeKosky ST ((2000) ) Psychotic symptoms in Alzheimer’s disease are not associated with more severe neuropathologic features. Int Psychogeriatr 12: , 547–558. |
[16] | Ballard CG , Jacoby R , Del Ser T , Khan MN , Munoz DG , Holmes C , Nagy Z , Perry EK , Joachim C , Jaros E , O’Brien JT , Perry RH , McKeith IG ((2004) ) Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with Lewy bodies. Am J Psychiatry 161: , 843–849. |
[17] | Koppel J , Acker C , Davies P , Lopez OL , Jimenez H , Azose M , Greenwald BS , Murray PS , Kirkwood CM , Kofler J , Sweet RA ((2014) ) Psychotic Alzheimer’s disease is associated with gender-specific tau phosphorylation abnormalities. Neurobiol Aging 35: , 2021–2028. |
[18] | Skogseth R , Mulugeta E , Jones E , Ballard C , Rongve A , Nore S , Alves G , Aarsland D ((2008) ) Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord 25: , 559–563. |
[19] | Ismail Z , Nguyen MQ , Fischer CE , Schweizer TA , Mulsant BH ((2012) ) Neuroimaging of delusions in Alzheimer’s disease. Psychiatry Res 202: , 89–95. |
[20] | Murray PS , Kumar S , Demichele-Sweet MA , Sweet RA ((2014) ) Psychosis in Alzheimer’s disease. Biol Psychiatry 75: , 542–552. |
[21] | Rosenberg PB , Nowrangi MA , Lyketsos CG ((2015) ) Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits?. Mol Aspects Med 43-44: , 25–37. |
[22] | Ting WK , Fischer CE , Millikin CP , Ismail Z , Chow TW , Schweizer TA ((2015) ) Grey matter atrophy in mild cognitive impairment / early Alzheimer disease associated with delusions: A voxel-based morphometry study. Curr Alzheimer Res 12: , 165–172. |
[23] | Fischer CE , Ting WK , Millikin CP , Ismail Z , Schweizer TA , Alzheimer Disease Neuroimaging Initiative ((2015) ) Gray matter atrophy in patients with mild cognitive impairment/Alzheimer’s disease over the course of developing delusions. Int J Geriatr Psychiatry, doi: 10.1002/gps.4291 |
[24] | Steinberg M , Hess K , Corcoran C , Mielke MM , Norton M , Breitner J , Green R , Leoutsakos J , Welsh-Bohmer K , Lyketsos C , Tschanz J ((2014) ) Vascular risk factors and neuropsychiatric symptoms in Alzheimer’s disease: The Cache County Study. Int J Geriatr Psychiatry 29: , 153–159. |
[25] | Fujishima M , Maikusa N , Nakamura K , Nakatsuka M , Matsuda H , Meguro K ((2014) ) Mild cognitive impairment, poor episodic memory, and late-life depression are associated with cerebral cortical thinning and increased white matter hyperintensities. Front Aging Neurosci 6: , 306. |
[26] | Beekly DL , Ramos EM , Lee WW , Deitrich WD , Jacka ME , Wu J , Hubbard JL , Koepsell TD , Morris JC , Kukull WA , Centers NIAAsD ((2007) ) The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord 21: , 249–258. |
[27] | Pfeffer RI , Kurosaki TT , Harrah CH Jr , Chance JM , Filos S ((1982) ) Measurement of functional activities in older adults in the community. J Gerontol 37: , 323–329. |
[28] | Folstein MF , Folstein SE , McHugh PR ((1975) ) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[29] | Hughes CP , Berg L , Danziger WL , Coben LA , Martin RL ((1982) ) A new clinical scale for the staging of dementia. Br J Psychiatry 140: , 566–572. |
[30] | Braak H , Braak E ((1991) ) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: , 239–259. |
[31] | Mirra SS , Heyman A , McKeel D , Sumi SM , Crain BJ , Brownlee LM , Vogel FS , Hughes JP , van Belle G , Berg L ((1991) ) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41: , 479–486. |
[32] | Hyman BT , Trojanowski JQ ((1997) ) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56: , 1095–1097. |
[33] | Nagy Z , Yilmazer-Hanke DM , Braak H , Braak E , Schultz C , Hanke J ((1998) ) Assessment of the pathological stages of Alzheimer’s disease in thin paraffin sections: A comparative study. Dement Geriatr Cogn Disord 9: , 140–144. |
[34] | McKeith IG , Dickson DW , Lowe J , Emre M , O’Brien JT , Feldman H , Cummings J , Duda JE , Lippa C , Perry EK , Aarsland D , Arai H , Ballard CG , Boeve B , Burn DJ , Costa D , Del Ser T , Dubois B , Galasko D , Gauthier S , Goetz CG , Gomez-Tortosa E , Halliday G , Hansen LA , Hardy J , Iwatsubo T , Kalaria RN , Kaufer D , Kenny RA , Korczyn A , Kosaka K , Lee VM , Lees A , Litvan I , Londos E , Lopez OL , Minoshima S , Mizuno Y , Molina JA , Mukaetova-Ladinska EB , Pasquier F , Perry RH , Schulz JB , Trojanowski JQ , Yamada M , Consortium on DLB ((2005) ) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 65: , 1863–1872. |
[35] | Roman GC ((1987) ) Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA 258: , 1782–1788. |
[36] | Caplan LR ((1995) ) Binswanger’s disease–revisited. Neurology 45: , 626–633. |
[37] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[38] | Kaufer DI , Cummings JL , Ketchel P , Smith V , MacMillan A , Shelley T , Lopez OL , DeKosky ST ((2000) ) Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 12: , 233–239. |
[39] | Weamer EA , Emanuel JE , Varon D , Miyahara S , Wilkosz PA , Lopez OL , Dekosky ST , Sweet RA ((2009) ) The relationship of excess cognitive impairment in MCI and early Alzheimer’s disease to the subsequent emergence of psychosis. Int Psychogeriatr 21: , 78–85. |
[40] | Jacobson SA , Morshed T , Dugger BN , Beach TG , Hentz JG , Adler CH , Shill HA , Sabbagh MN , Belden CM , Sue LI , Caviness JN , Hu C , Arizona Parkinson’s Disease Consortium ((2014) ) Plaques and tangles as well as Lewy-type alpha synucleinopathy are associated with formed visual hallucinations. Parkinsonism Relat Disord 20: , 1009–1014. |
[41] | Bidzan M , Bidzan L , Pachalska M ((2014) ) Neuropsychiatric symptoms in patients with Alzheimer’s disease with a vascular component. Ann Agric Environ Med 21: , 412–415. |
[42] | Kertesz A , Clydesdale S ((1994) ) Neuropsychological deficits in vascular dementia vs Alzheimer’s disease. Frontal lobe deficits prominent in vascular dementia. Arch Neurol 51: , 1226–1231. |
[43] | Monsell SE , Mock C , Roe CM , Ghoshal N , Morris JC , Cairns NJ , Kukull W ((2013) ) Comparison of symptomatic and asymptomatic persons with Alzheimer disease neuropathology. Neurology 80: , 2121–2129. |
Figures and Tables
Fig.1
Distribution of clinically diagnosed (cAD) and neuropathologically confirmed (npAD) Alzheimer’s disease subjects with neuropathological data into psychotic groups.
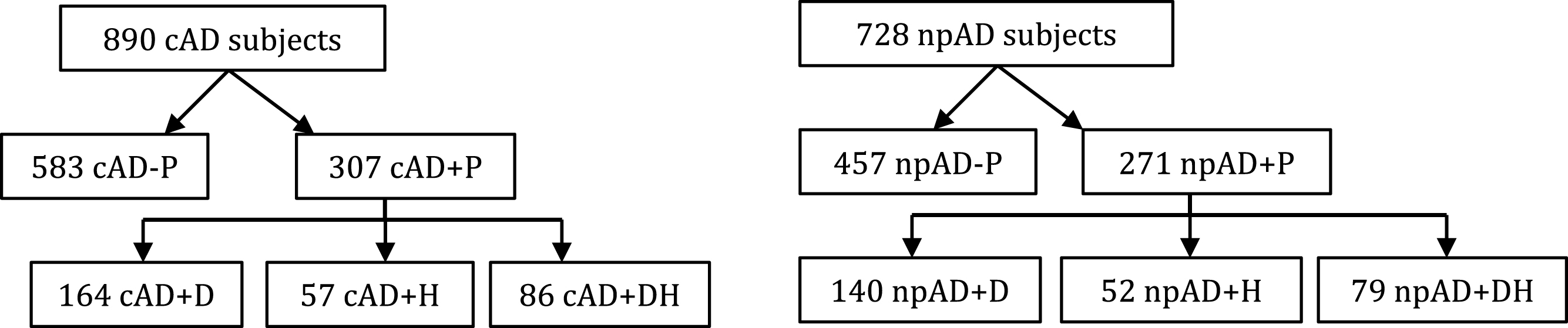
Table 1
Demographics, clinical, pathological correlates, and vascular risk factors of clinically diagnosed AD patients with and without psychosis
| Variable | Psychosis status | |||||||||
| cAD–P | cAD+P | cAD+D | cAD+H | cAD+DH | ||||||
| n = 583 (66%) | n = 307 (34%) | n = 164 (53%) | n = 58 (19%) | n = 86 (28%) | ||||||
| N/mean | % /SD | N/mean | % /SD | N/mean | % /SD | N/mean | % /SD | N/mean | % /SD | |
| Age of death | 81.3 | 10.0 | 80.1 | 10.5 | 80.7 | 10.9 | 79.8 | 9.6 | 79.4 | 10.2 |
| Male | 336 | 57.5% | 158 | 51.5% | 83 | 50.6% | 30 | 51.7% | 46 | 51.7% |
| Education (y) | 15.0 | 3.3 | 14.9 | 3.2 | 15.1 | 3.1 | 15.1 | 2.5 | 14.3 | 3.8 |
| Disease duration (y) | 9.6 | 4.3 | 10.3 * | 4.3 | 10.3 * | 4.2 | 9.79 | 4.5 | 10.6 * | 4.4 |
| Years between last | 1.45 | 1.51 | 1.78 * | 1.50 | 2.05 * | 1.55 | 1.28 | 1.15 | 1.63 | 1.50 |
| clinical visit and death | ||||||||||
| CDR | * | * | * | |||||||
| Severe impairment | 230 | 39.4% | 125 | 40.7% | 46 | 28.0% | 33 | 56.9% | 46 | 53.5% |
| Moderate impairment | 186 | 31.8% | 112 | 36.5% | 60 | 36.6% | 19 | 32.8% | 34 | 39.5% |
| Mild impairment | 135 | 23.1% | 61 | 19.9% | 50 | 30.5% | 5 | 8.6% | 6 | 7.0% |
| Questionable impairment | 33 | 5.7% | 9 | 2.9% | 8 | 4.9% | 1 | 1.7% | 0 | 0.0% |
| No impairment | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| MMSE | 14.3 | 8.1 | 13.5 | 7.6 | 15.8 | 7.3 | 10.5 * | 8.1 | 10.2 * | 6.0 |
| FAQ | 26.8 | 5.3 | 27.7 | 4.0 | 26.4 | 4.9 | 29.0 * | 2.7 | 28.8 * | 2.4 |
| Braak Stage | * | * | * | |||||||
| 6 | 265 | 45.6% | 164 | 53.6% | 90 | 54.9% | 27 | 47.4% % | 48 | 55.8% |
| 5 | 137 | 23.6% | 89 | 29.1% | 41 | 25.0% | 22 | 38.6% | 26 | 30.2% |
| 4 | 78 | 13.4% | 32 | 10.5% | 20 | 12.2% | 4 | 7.0% | 8 | 9.3% |
| 3 | 36 | 6.2% | 7 | 2.3% | 4 | 2.4% | 3 | 5.3% | 0 | 0% |
| 2 | 27 | 4.6% | 8 | 2.6% | 5 | 3.0% | 0 | 0% | 3 | 3.5% |
| 1 | 26 | 4.5% | 2 | 0.7% | 2 | 1.2% | 0 | 0% | 0 | 0% |
| Criteria not met | 12 | 2.1% | 4 | 1.3% | 2 | 1.2% | 1 | 1.8% | 1 | 1.2% |
| NIA Reagan | * | * | * | * | ||||||
| High | 396 | 68.2% | 244 | 79.7% | 129 | 78.7% | 47 | 82.5% | 69 | 80.2% |
| Intermediate | 92 | 15.8% | 39 | 12.7% | 23 | 14.0% | 6 | 10.5% | 10 | 11.6% |
| Low | 33 | 5.7% | 9 | 2.9% | 4 | 2.4% | 1 | 1.8% | 4 | 4.7% |
| Not met | 60 | 10.3% | 14 | 4.6% | 8 | 4.9% | 3 | 5.3% | 3 | 3.5% |
| CERAD | * | * | * | * | ||||||
| Definite AD | 389 | 70.3% | 221 | 82.8% | 122 | 84.7% | 42 | 82.4% | 58 | 79.5% |
| Probable AD | 73 | 13.2% | 26 | 9.7% | 10 | 6.9% | 7 | 13.7% | 9 | 12.3% |
| Possible AD | 28 | 5.1% | 9 | 3.4% | 5 | 3.5% | 0 | 0% | 4 | 5.5% |
| Criteria not met | 63 | 11.4% | 11 | 4.1% | 7 | 4.9% | 2 | 3.9% | 2 | 2.7% |
| Neuritic Plaques | * | * | ||||||||
| Frequent | 393 | 67.3% | 228 | 74.3% | 121 | 73.8% | 44 | 75.9% | 64 | 74.4% |
| Moderate | 104 | 17.8% | 61 | 19.9% | 32 | 19.5% | 11 | 19.0% | 18 | 20.9% |
| Sparse | 29 | 5.0% | 9 | 2.9% | 5 | 3.0% | 1 | 1.7% | 3 | 3.5% |
| None | 58 | 9.9% | 9 | 2.9% | 6 | 3.7% | 2 | 3.4% | 1 | 1.2% |
| Lewy Bodies | * | * | ||||||||
| Diffuse neocortical | 67 | 12.6% | 48 | 17.4% | 24 | 15.6% | 11 | 21.2% | 13 | 18.3% |
| Limbic transitional | 52 | 9.8% | 35 | 12.7% | 21 | 13.6% | 5 | 9.6% | 9 | 12.7% |
| Brainstem type | 16 | 3.0% | 15 | 5.4% | 10 | 6.5% | 2 | 3.8% | 3 | 4.2% |
| No Lewy bodies | 397 | 74.6% | 178 | 64.5% | 99 | 64.3% | 34 | 65.4% | 46 | 64.8% |
| Gross/ Microscopic | ||||||||||
| vascular pathology | ||||||||||
| Yes | 581 | 99.5% | 305 | 99.3% | 163 | 99.4% | 57 | 98.3% | 86 | 100% |
| No | 3 | 0.5% | 2 | 0.7% | 1 | .6% | 1 | 1.7% | 0 | 0% |
| Microinfarcts | ||||||||||
| Yes | 89 | 15.3% | 57 | 18.6% | 32 | 19.5% | 9 | 15.5% | 17 | 19.8% |
| No | 494 | 84.7% | 250 | 81.4% | 132 | 80.5% | 49 | 84.5% | 69 | 80.2% |
| Lacunes | ||||||||||
| Yes | 110 | 18.9% | 52 | 16.9% | 26 | 15.9% | 8 | 13.8% | 18 | 20.9% |
| No | 472 | 81.1% | 255 | 83.1% | 138 | 84.1% | 50 | 86.2% | 68 | 79.1% |
| Hippocampal sclerosis | ||||||||||
| Yes | 69 | 14.1% | 35 | 13.6% | 16 | 11.6% | 10 | 19.2% | 9 | 13.0% |
| No | 416 | 84.9% | 220 | 85.3% | 120 | 87.0% | 42 | 80.8% | 59 | 85.5% |
| Hemorrhages | ||||||||||
| Yes | 38 | 6.5% | 14 | 4.6% | 8 | 4.9% | 2 | 3.4% | 4 | 4.7% |
| No | 544 | 93.5% | 293 | 95.4% | 156 | 95.1% | 56 | 96.6% | 82 | 95.3% |
| Subcortical Arteriosclerotic | * | * | ||||||||
| Leukoencephalopathy | ||||||||||
| Yes | 76 | 13.1% | 63 | 20.6% | 31 | 19.0% | 11 | 19.0% | 21 | 24.4% |
| No | 506 | 86.9% | 243 | 79.4% | 132 | 81.0% | 47 | 81.0% | 65 | 75.6% |
| Smoker | ||||||||||
| No | 313 | 55.7% | 172 | 56.6% | 90 | 55.2% | 35 | 61.4% | 48 | 56.5% |
| Yes | 249 | 44.3% | 132 | 43.4% | 73 | 44.8% | 22 | 38.6% | 37 | 43.5% |
| Total years smoked | 25.3 | 17.6 | 27.7 | 18.7 | 27.7 | 18.8 | 27.3 | 18.1 | 28.0 | 19.4 |
| Packs/day | * | |||||||||
| 1 cig -<1/2 pack | 71 | 29.6% | 28 | 23.5% | 14 | 21.5% | 2 | 11.8% | 12 | 32.6% |
| 1/2 -<1 pack | 82 | 34.2% | 48 | 40.3% | 30 | 46.2% | 4 | 23.5% | 14 | 37.8% |
| 1 -<1 1/2 pack | 50 | 20.8% | 26 | 21.8% | 15 | 23.1% | 5 | 29.4% | 6 | 16.2% |
| 1 1/2 -<2 packs | 15 | 6.3% | 9 | 7.6% | 4 | 6.2% | 2 | 11.8% | 3 | 8.1% |
| ≥2 packs | 19 | 7.9% | 8 | 6.7% | 2 | 3.1% | 4 | 23.5% | 2 | 5.4% |
| Age quit smoking | 45.5 | 16.3 | 48.1 | 18.0 | 46.4 | 19.3 | 54.1 * | 17.2 | 47.9 | 15.4 |
| Hypertension | * | * | ||||||||
| Absent | 314 | 56.2% | 139 | 46.6% | 77 | 47.8% | 26 | 47.3 | 37 | 44.5% |
| Active | 245 | 43.8% | 159 | 53.3% | 84 | 52.2% | 29 | 52.7 | 46 | 55.4% |
| Hypercholesterolemia | ||||||||||
| Absent | 329 | 58.8% | 156 | 52.5% | 86 | 53.8% | 29 | 55.8% | 42 | 48.8% |
| Active | 231 | 41.25% | 141 | 47.5% | 74 | 46.3% | 23 | 44.2% | 44 | 51.2% |
| Diabetes | * | |||||||||
| Absent | 538 | 92.8% | 271 | 89.1% | 145 | 90.1% | 55 | 94.8% | 72 | 83.7% |
| Active | 42 | 7.2% | 33 | 10.9% | 16 | 9.9% | 3 | 5.2% | 14 | 16.3% |
cAD-P, never psychotic; cAD+P, psychosis; cAD+D, delusional psychosis; cAD+H, hallucinatory psychosis; cAD+DH delusional and hallucinatory psychosis. *Indicates significant difference compared to cAD-P, two-tailed, p < 0.05.
Table 2
Demographics, clinical, pathological correlates, and vascular risk factors of neuropathologically definite AD patients with and without psychosis
| Variable | Psychosis status | |||||||||
| npAD–P | npAD+P | npAD+D | npAD+H | npAD+DH | ||||||
| n = 457 (63%) | n = 271 (37%) | n = 140 (52%) | n = 52 (19%) | n = 79 (29%) | ||||||
| N/mean | % /SD | N/mean | % /SD | N/mean | % /SD | N/mean | % /SD | N/mean | % /SD | |
| Age of death | 80.0 | 10.3 | 78.9 | 10.4 | 79.6 | 10.7 | 78.0 | 10.3 | 78.5 | 10.0 |
| Male | 260 | 56.9% | 154 | 57% | 76 | 54.3% | 29 | 55.8% | 50 | 63.3% |
| Education (y) | 15.3 | 3.2 | 15.0 | 3.2 | 15.3 | 3.0 | 15.0 | 2.8 | 14.7 | 3.6 |
| Disease duration (y) | 9.8 | 3.9 | 9.9 | 3.9 | 10.1 | 4.0 | 9.6 | 4.3 | 9.7 | 3.7 |
| Years between last | 1.54 | 1.58 | 1.81 * | 1.50 | 2.04 * | 1.55 | 1.29 | 1.14 | 1.75 | 1.52 |
| clinical visit and death | ||||||||||
| CDR | * | * | * | |||||||
| Severe impairment | 196 | 42.9% | 113 | 41.9% | 40 | 28.6% | 32 | 61.5% | 41 | 51.9% |
| Moderate impairment | 148 | 32.4% | 103 | 38.1% | 61 | 43.6% | 15 | 28.8% | 28 | 35.4% |
| Mild impairment | 91 | 19.9% | 46 | 17.0% | 34 | 24.3% | 2 | 3.8% | 10 | 12.7% |
| Questionable impairment | 22 | 4.8% | 8 | 3.0% | 5 | 3.6% | 3 | 5.8% | 0 | 0.0% |
| No impairment | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| MMSE | 13.0 | 8.2 | 13.0 | 7.2 | 14.8 | 6.9 | 9.9 * | 8.1 | 11.0 * | 6.2 |
| FAQ | 27.1 | 5.2 | 27.8 | 4.0 | 26.8 | 4.6 | 28.4 * | 4.1 | 28.9 * | 2.3 |
| Braak Stage | ||||||||||
| 6 | 271 | 59.3% | 160 | 59.3% | 87 | 62.1% | 31 | 59.6% | 43% | 54.4% |
| 5 | 130 | 28.4% | 90 | 33.3% | 42 | 30.0% | 18 | 34.6% | 30% | 38.0% |
| 4 | 43 | 9.4% | 13 | 4.8% | 6 | 4.3% | 1 | 1.9% | 6% | 7.6% |
| 3 | 10 | 2.2% | 2 | 0.7% | 1 | .8% | 1 | 1.9% | 0% | 0% |
| 2 | 2 | 0.4% | 3 | 1.1% | 2 | 1.4% | 1 | 1.9% | 0% | 0% |
| 1 | 0 | 0% | 1 | 0.4% | 1 | 0.7% | 0 | 0% | 0% | 0% |
| Criteria not met | 1 | 0.2% | 1 | 0.4% | 1 | 0.7% | 0 | 0% | 0% | 0% |
| NIA Reagan | ||||||||||
| High | 412 | 90.2% | 252 | 93.3% | 131 | 93.6% | 49 | 94.2% | 73 | 92.4% |
| Intermediate | 42 | 9.2% | 12 | 4.4% | 7 | 5.0% | 1 | 1.9% | 4 | 5.1% |
| Low | 1 | 0.2% | 2 | 0.7% | 1 | 0.7% | 1 | 1.9% | 0 | 0.0% |
| Not met | 2 | 0.4% | 4 | 1.5% | 1 | 0.7% | 1 | 1.9% | 2 | 2.5% |
| Neuritic Plaques | ||||||||||
| Frequent | 425 | 93.0% | 251 | 93.0% | 131 | 93.6% | 48 | 92.3% | 73 | 92.4% |
| Moderate | 31 | 6.8% | 19 | 7.0% | 9 | 6.4% | 4 | 7.7% | 6 | 7.6% |
| Sparse | 1 | 0.2% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| None | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Lewy Bodies | * | * | * | |||||||
| Diffuse neocortical | 62 | 15.2% | 61 | 25.8% | 24 | 18.8% | 16 | 34.8% | 21 | 33.3% |
| Limbic transitional | 46 | 11.3% | 30 | 12.7% | 19 | 14.8% | 3 | 6.5% | 8 | 12.7% |
| Brainstem type | 13 | 3.2% | 11 | 4.7% | 7 | 5.5% | 2 | 4.3% | 2 | 3.2% |
| No LB | 287 | 70.3% | 134 | 56.8% | 78 | 60.9% | 25 | 54.3% | 32 | 50.8% |
| Subcortical Arteriosclerotic | * | * | ||||||||
| Leukoencephalopathy | ||||||||||
| Yes | 67 | 14.7% | 64 | 23.8% | 29 | 20.9% | 12 | 23.1% | 23 | 29.1% |
| No | 388 | 85.3% | 205 | 76.2% | 110 | 79.1% | 40 | 76.9% | 56 | 70.9% |
| Smoker | ||||||||||
| No | 258 | 59.0% | 140 | 52.4% | 75 | 54.0% | 29 | 56.9% | 37 | 47.4% |
| Yes | 179 | 41.0% | 127 | 47.6% | 64 | 46.0% | 22 | 43.1% | 41 | 52.6% |
| Total years smoked | 24.5 | 17.8 | 27.4 | 17.0 | 27.6 | 17.5 | 25.3 | 17.0 | 28.4 | 16.6 |
| Packs/day | ||||||||||
| 1 cig -<1/2 pack | 48 | 27.6% | 26 | 23.2% | 12 | 21.4% | 3 | 16.7% | 11 | 28.9% |
| 1/2 -<1 pack | 61 | 35.1% | 45 | 40.2% | 25 | 44.6% | 5 | 27.8% | 15 | 39.5% |
| 1 -<1 1/2 pack | 41 | 23.6% | 27 | 24.1% | 13 | 23.2% | 6 | 33.3% | 8 | 21.1% |
| 1 1/2 -<2 packs | 8 | 4.6% | 7 | 6.2% | 4 | 7.1% | 1 | 5.6% | 2 | 5.3% |
| ≥2 packs | 14 | 8.0% | 7 | 6.2% | 2 | 3.6% | 3 | 16.7% | 2 | 5.3% |
| Age quit smoking | 45.4 | 16.4 | 48.7 | 16.8 | 46.8 | 17.6 | 54.3 * | 17.9 | 48.3 | 14.3 |
| Hypertension | * | |||||||||
| Absent | 246 | 56.6% | 124 | 47.7% | 64 | 47.1% | 24 | 49.0% | 37 | 48.7% |
| Active | 189 | 43.4% | 136 | 52.3% | 72 | 52.9% | 25 | 51.0% | 39 | 51.3% |
| Hypercholesterolemia | ||||||||||
| Absent | 254 | 58.4% | 135 | 52.7% | 72 | 53.3% | 25 | 54.3% | 39 | 51.3% |
| Active | 181 | 41.6% | 121 | 47.3% | 63 | 46.7% | 21 | 45.7% | 37 | 48.7% |
| Diabetes | * | |||||||||
| Absent | 416 | 91.4% | 233 | 87.3% | 120 | 87.6% | 48 | 92.3% | 66 | 83.5% |
| Active | 39 | 8.6% | 34 | 12.7% | 17 | 12.4% | 4 | 7.7% | 13 | 16.5% |
npAD–P, never psychotic; npAD+P, psychosis; npAD+D, delusional psychosis; npAD+H hallucinatory psychosis; npAD+DH, delusional and hallucinatory psychosis. *Indicates significant difference compared to npAD-P, two-tailed, p < 0.05.
Table 3
Test statistics for significant variables comparing clinically diagnosed AD patients (cAD) with and without psychosis. The gray cells represent non-significant associations
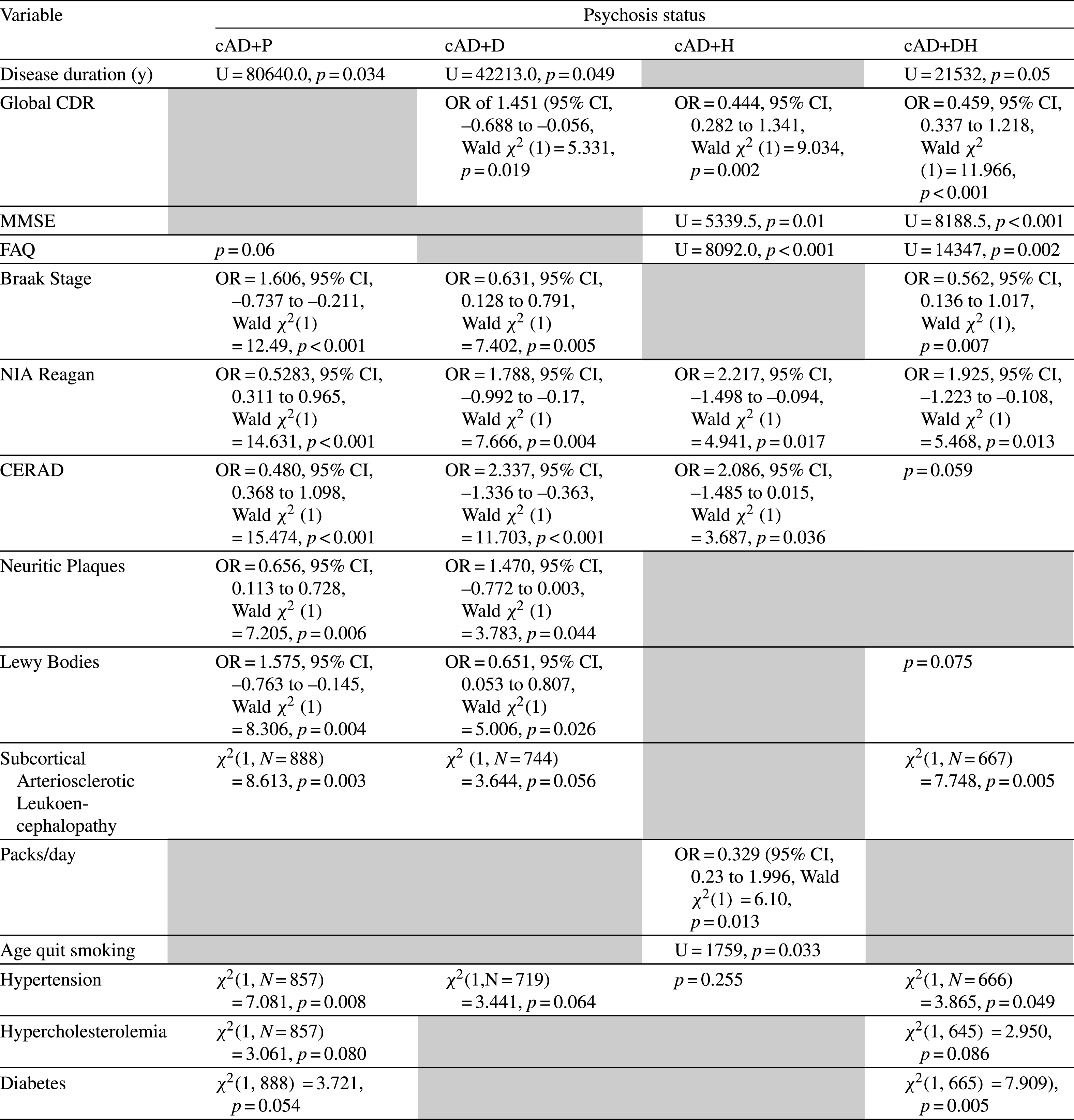 |
Table 4
Results of statistical analysis comparing neuropathologically diagnosed AD patients (npAD) with and without psychosis. The gray cells represent non-significant associations
 |



