APOE-ɛ4 Carrier Status and Donepezil Response in Patients with Alzheimer’s Disease
Abstract
Background: Previous studies have investigated associations between apolipoprotein E (APOE)-ɛ4 allele status and acetylcholinesterase inhibitor treatment response in patients with Alzheimer’s disease. The ability to draw definitive conclusions regarding the effect of APOE-ɛ4 genotype on treatment response has been hindered by inconsistent results among studies and methodological limitations that restrict interpretation of study findings.
Objective: To determine whether APOE-ɛ4 carrier status influences the magnitude of change in 13-item Alzheimer’s Disease Assessment Scale−Cognitive Subscale (ADAS-cog) score associated with acetylcholinesterase inhibitor treatment (i.e., donepezil).
Methods: Analyses were performed using pooled data from the donepezil and placebo treatment arms of three consecutive, similarly designed, 12-week, multi-national, randomized clinical studies that enrolled patients with mild-to-moderate Alzheimer’s disease. Correlations between APOE-ɛ4 carrier status and ADAS-cog scores were evaluated using analysis of covariance.
Results: No appreciable interaction between donepezil response and APOE-ɛ4 carrier status or copy number was detected. Both carriers and non-carriers of APOE-ɛ4 who received donepezil experienced significant improvements from baseline in ADAS-cog score versus placebo (p < 0.05). Change from baseline to final observation in the donepezil treatment group was – 2.95 for APOE-ɛ4 carriers and – 4.09 for non-carriers (p = 0.23). In contrast, non-carriers of APOE-ɛ4 in the placebo treatment group exhibited a greater improvement from baseline versus carriers (–2.38 versus – 0.60, p = 0.05).
Conclusion: Within this population, APOE genotype had no statistically significant effect on cognitive response to donepezil treatment; however, APOE-ɛ4 allele status was associated with a difference in the magnitude of the change in ADAS-cog of placebo-treated patients.
INTRODUCTION
The association between the ɛ4 allele of the gene encoding apolipoprotein E (APOE) and risk for late-onset familial and sporadic forms of Alzheimer’s disease (AD) was first established more than 20 years ago [1–4]. Accumulated clinical data and epidemiologic evidence have since implicated APOE-ɛ4 as playing an etiologic role in as many as 50% of AD cases in the United States [5]. There is an apparent dosage effect related to the APOE-ɛ4 allele such that homozygosity confers a substantially greater risk for AD than does carrying a single copy of the ɛ4 allele [6]. Moreover, the number of APOE-ɛ4 alleles is inversely correlated with age of AD presentation in patients with late-onset disease [7]. The APOE-ɛ4 allele is present in an estimated 13% of the general population worldwide, with regional frequencies varying from a low of 7% in India to a high of 22% in Oceania [8]. As would be expected given the disease linkage, the APOE-ɛ4 allele is more pervasive among patients with AD compared with the general population [3, 9].
Although clearly influential in the pathogenesis of AD, the effect of APOE genotype on an individual’s response to treatment is less well understood. Clinical trial data suggest that the presence or absence of APOE-ɛ4 contributes to therapeutic response for a range of AD drugs, both approved (e.g., acetylcholinesterase inhibitors) and investigational (e.g., thiazolidinediones, immunotherapy agents) [10–12]. As the most commonly prescribed class of medication used to treat AD, acetylcholinesterase inhibitors have been of particular interest in this respect. Multiple studies have examined the effect of APOE-ɛ4 carrier status on treatment response to donepezil, an acetylcholinesterase inhibitor that is frequently used in clinical practice. The results of these analyses have been mixed, with several studies suggesting that APOE-ɛ4 carrier status does not affect treatment response [13–15], yet other analyses indicating that the presence of the APOE-ɛ4 allele is associated with greater treatment-related improvements [16, 17] (Table 1). A similar inconsistent pattern across studies has been observed with other acetylcholinesterase inhibitors. There is some evidence for a better response to treatment among carriers of the APOE-ɛ4 allele versus non-carriers [18]; however, a few studies have suggested the opposite relationship [19–21], and the majority of assessments have shown no correlation between APOE-ɛ4 carrier status and treatment response [22–26]. In many cases, the small sample size and the exploratory nature of the analyses make interpretation of the results difficult. In addition, disparate outcomes among these studies can be attributed to a multitude of factors, including differences in characteristics of the study population (e.g., baseline AD severity, duration of treatment, country), allele distribution, study drug properties, outcome measure, and other factors.
To better understand the possible link between treatment response and APOE-ɛ4 carrier status, data were pooled from the donepezil and placebo treatment arms of three consecutive, comparable 12-week, multi-national clinical trials conducted in eight countries that together included more than 300 patients with mild-to-moderate AD who were treated with donepezil or placebo. Assessment of treatment response according to APOE-ɛ4 genotype was a pre-specified subgroup analysis in each of the included studies. The resulting dataset has yielded the largest and most geographically diverse evaluation of a possible correlation between APOE-ɛ4 carrier status and donepezil treatment response available to date.
MATERIALS AND METHODS
Patients
Adult men and women aged 55 to 90 years who were diagnosed with mild-to-moderate AD, as defined by the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria for probable AD [27], were eligible for inclusion in the studies. Study participants must have scored between 10 and 24 (inclusive) on the Mini-Mental State Examination (MMSE), 10 or below on the Cornell Scale for Depression in Dementia, and 4 or below on the Modified Hachinski Ischemic Scale at the time of the first screening visit. Computed tomography or magnetic resonance imaging must have been performed for each subject within 36 months prior to randomization to exclude alternative causes for dementia. With the exception of AD, patients were to be in generally good health as indicated by medical history, findings at physical examination, vital sign measurements, results from laboratory profile, and results from 12-lead electrocardiography. Patients who had a history of donepezil treatment failure; who were receiving or had received medication for the treatment of AD within 60 days prior to the screening visit; who were currently taking or had taken a vitamin K antagonist within 30 days of the screening visit; or who had clinically significant disease, including a history of neurologic disorders (e.g., Parkinson’s disease, multi-infarct or vascular dementia, Huntington’s disease, etc.), were not eligible to participate in the study. All study participants provided written informed consent.
Study design
Data from three phase 2, 12-week, multi-national, randomized, double-blind, parallel-group clinical trials designed to evaluate the safety and efficacy of investigational agents for the treatment of mild-to-moderate AD conducted from 2009 to 2011 were pooled for this analysis (Table 2) [28–30]. Eight countries enrolled patients in 1 or more of the studies. Participating countries included Bulgaria, the Czech Republic, Russia, Slovakia, South Africa, Ukraine, the United Kingdom, and the United States. The trials had the same study design, with a screening period of up to 28 days followed by randomization to treatment with a low or high dose of an investigational drug (ABT-126, ABT-288, or ABT-384), placebo, or active control (donepezil) for 12 weeks, and a subsequent 30-day follow-up period (Fig. 1). Primary efficacy results from all three studies revealed a trend toward or a statistically significant improvement in the 13-item Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) among patients in the donepezil treatment arm [28–30]. Patients assigned to donepezil treatment were given a 5-mg dose once daily for the first 4 weeks of the treatment phase and a 10-mg dose once daily for the remaining 8 weeks, consistent with the dosing instructions in the product label. Study protocols received Institutional Review Board approval and study procedures were conducted in compliance with the principles of the Declaration of Helsinki and guidelines for Good Clinical Practice.
All three studies included protocol-specified, interim futility analyses to determine whether the experimental drug treatment arms were likely to demonstrate clinical benefit according to criteria specified in the Data Monitoring Committee charter. For two of the studies (NCT01018875 and NCT01137526), the criteria for futility were met and the studies were prematurely terminated; treatment was discontinued for all treatment arms and patients reported to their respective study sites for final study visits. Close to two-thirds of patients had completed the studies at the time of termination (Table 2) [29, 30].
Efficacy measures
Efficacy assessments were performed at 4, 8, and 12 weeks after treatment initiation. The ADAS-cog total score was generated by summing the component 13 items, which included word recall, commands, constructional praxis, delayed-word recall, naming objects and fingers, ideational praxis, orientation, word recognition, remembering test instructions, number cancellation, comprehension of spoken language, spoken-language ability, and word-finding difficulty. Total scores for the 13-item ADAS-cog scale range from 0 to 85, with a higher score indicating greater impairment.
Sample collection and APOE-ɛ4 genotyping
Patients had the option of providing a blood sample for pharmacogenetic analysis; written informed consent was obtained for those who participated. One 4-mL whole blood sample for DNA isolation was collected into an ethylenediaminetetraacetic acid-containing tube from each consenting patient during the screening period and stored at – 20°C until DNA extraction. DNA was isolated from whole blood using Qiagen reagent kits (Qiagen Inc, Valencia, CA) and applied to an AutoGenprep 3000 (AutoGen Inc, Holliston, MA) automated DNA extraction instrument.
Genotypes were determined for the followingnucleotide APOE polymorphisms: 3937T>C (rs429358) and 4075C>T (rs7412). All genotypes were determined using the Pyrosequencing detectionmethod (Pyrosequencing Inc, Westborough, MA)following standard operating procedures. Primer sequences for pyrosequencing were as follows: 5′-GGACATGGAGGACGTG-3′ (APOE 3937T>C) and 5′– CCGATGACCTGCAGAAG-3′ (APOE 4075C>T). Primer sequences used for allele-specific PCR included 5′-AAGGAGCTGCAGGCGGCGCAGG-3′ (forward) and 5′-GGATGGCGCTGAGGCCGCGC-3′ (reverse).
Statistical analyses
Data for the intent-to-treat (ITT) and completer populations of the donepezil and placebo-treatment groups were pooled from the three studies. The ITT population included all randomized patients who received at least 1 dose of study drug (n = 391). For those individuals who did not complete the study, efficacy evaluations were performed using the last-observation-carried-forward method. The completer population consisted of patients in the ITT population who completed the full treatment period and had data available for the 12-week efficacy assessment (n = 287). The primary analyses were performed using the ITT dataset. Results from the completer analysis were reported if the conclusions differed from those of the ITT analysis.
Evaluation of treatment response according to APOE-ɛ4 genotype was pre-specified in the study protocols. For the assessment of APOE-ɛ4 carrier status on treatment response, least square (LS) mean change from baseline to final evaluation in the ADAS-cog total score was analyzed using analysis of covariance with effects of age, treatment, study site, APOE-ɛ4 allele status, APOE-ɛ4-by-treatment interaction and covariate of baseline ADAS-cog total score. To examine the effect of APOE-ɛ4 copy number on treatment response, the same model was applied with APOE-ɛ4 allele status replaced by APOE-ɛ4 copy number.
RESULTS
Patient characteristics
In the ITT population, demographics and baseline characteristics were comparable between the pooled placebo and pooled donepezil groups (Table 3). Approximately 57% of the patients were female and nearly two-thirds had not previously received an agent approved for the treatment of AD. Mean baseline MMSE scores were approximately 19, and MMSE and ADAS-cog scores were similar between patients assigned to placebo or donepezil. APOE-ɛ4 genotype data were available for 170 out of 197 patients who received placebo and 165 out of 194 patients who received donepezil. Homozygosity for the APOE-ɛ4 allele was more common among placebo-treated patients versus donepezil-treated patients (12.9% and 5.5% , respectively).
Influence of APOE genotype on change from baseline to final observation in ADAS-cog score for placebo- and donepezil-treated patients
Both APOE-ɛ4 carriers and non-carriers showed a statistically significant improvement (indicated by a decrease in ADAS-cog total score) with donepezil treatment. The LS mean difference between donepezil- and placebo-treated patients in ADAS-cog at the final evaluation was – 2.34 for carriers and – 1.71 for non-carriers (p = 0.01 and p = 0.05, respectively) in the ITT population (Fig. 2A). In the smaller completer population (n = 264), donepezil showed a statistically significant treatment effect in the APOE-ɛ4 carriers (n = 136, – 3.53; p < 0.001), but not among non-carriers (n = 128, – 1.62; p = 0.113). Overall, however, the interaction between APOE-ɛ4 carrier status and treatment effect on the ADAS-cog was not significant in either the ITT (p = 0.61) or completer (p = 0.20) populations.
Improvement from baseline in mean ADAS-cog score with donepezil treatment was comparable in carriers and non-carriers of the APOE-ɛ4 allele (Fig. 2B). In the ITT population, change from baseline to final observation in ADAS-cog was – 2.95 for APOE-ɛ4 carriers and – 4.09 for non-carriers (p = 0.23). Similar results were observed within the completer population. Interestingly, APOE-ɛ4 carrier status was associated with a difference in the magnitude of response on the ADAS-cog in placebo group. The LS mean change from baseline to final visit in ADAS-cog was significantly greater among APOE-ɛ4 non-carriers versus carriers (–2.38 and – 0.60, respectively; p = 0.05). This divergence increased over time (Fig. 3) and was more apparent within the completer population (–3.34 and 0.13, respectively; p = 0.002). Together, these results suggest that APOE-ɛ4 carrier status does not affect the magnitude of the improvement observed with donepezil treatment, but may be associated with a difference in the extent of the placebo response.
To rule out a possible influence of enrollment country on change in ADAS-cog score among APOE-ɛ4 non-carriers in the placebo treatment group, an analysis was performed using a statistical model that included the effect of study site within country, country, APOE-ɛ4 status, and covariates of subject age and baseline ADAS-cog score. The influence of country was determined to be insignificant, indicating that the results in the placebo group were not driven by outcomes from any single country.
APOE-ɛ4 copy number and ADAS-cog response
The influence of APOE-ɛ4 carrier status on the donepezil-placebo treatment difference and the change from baseline to final observation in donepezil- and placebo-treated patients was further investigated by subdividing the samples into specific genotype groups (i.e., non-carriers [n = 160], heterozygous [n = 144], and homozygous [n = 31] APOE-ɛ4 patients). Within the ITT population, a significant donepezil treatment effect was observed relative to placebo among patients with 0 or 1 copy of the APOE-ɛ4 allele (Fig. 4A). The difference in mean ADAS-cog score between patients treated with donepezil versus placebo was – 1.71 for non-carriers (p = 0.05) and – 2.32 for patients with 1 APOE-ɛ4 allele (p = 0.02). The treatment difference in patients homozygous for the APOE-ɛ4 allele was similar to that of APOE-ɛ4 heterozygotes (–2.31 and – 2.32, respectively), but did not achieve statistical significance, likely due to the comparatively small number of patients in the APOE-ɛ4 homozygote group. In the completer analysis, the only significant donepezil treatment effect was detected among carriers of 1 APOE-ɛ4 allele (–3.42; p = 0.004). Nonetheless, the interaction between APOE-ɛ4 copy number and treatment was not significant in either the ITT (p = 0.89) or completer (p = 0.54) populations.
Similar to the results described above that examined response to donepezil treatment in carriers and non-carriers, change from baseline to final observation in ADAS-cog scores of donepezil-treated patients was not influenced by the presence of 0, 1, or 2 APOE-ɛ4 alleles. Results from the ITT population are presented in Fig. 4B and are similar to those of the completer analysis. In the placebo group, however, LS mean change from baseline in ADAS-cog demonstrated less improvement among patients with 1 or 2 APOE-ɛ4 alleles versus non-carriers (Fig. 4B). This difference approached statistical significance for the comparison of 1 copy versus no copies of APOE-ɛ4 in the ITT population (between-group difference, 1.72; p = 0.08) and was statistically significant within the completer population (between-group difference, 3.15; p = 0.008). A significant difference in ADAS-cog score change from baseline was also observed in carriers of 2 APOE-ɛ4 alleles versus non-carriers in the completer analysis (between-group difference, 4.36; p = 0.008).
DISCUSSION
Pooled data from three consecutive, randomized, placebo-controlled, multi-national clinical trials conducted from 2009 to 2011 demonstrated that APOE-ɛ4 allele status has no statistically significant effect on the difference in treatment response between donepezil and placebo or the change from baseline to final observation in donepezil-treated patients as measured by ADAS-cog. Tests of interaction revealed no significant effect of either APOE-ɛ4 presence or copy number on donepezil efficacy, and comparable findings were observed in both the ITT and completer populations. The pooled dataset utilized for these analyses is the largest and most geographically diverse dataset in which the relationship between APOE-ɛ4 carrier status and donepezil treatment response has been examined.
The analyses performed in this assessment were prompted by the mixed results from previous studies that evaluated the effect of APOE genotype on response to donepezil or other acetylcholinesterase inhibitors. If donepezil studies are considered independently of other acetylcholinesterase inhibitor clinical trials (Table 1), a pattern emerges such that the larger studies (i.e., those that enrolled 100 or more patients) reported no difference in response to treatment in APOE-ɛ4 carriers versus non-carriers [13, 14], whereas smaller studies (i.e., those that enrolled approximately 50 patients or fewer) tended to indicate a correlation between outcomes and APOE-ɛ4 carrier status [15–17]. Our analysis, which included 165 patients who were treated with donepezil, is consistent with the findings of the larger studies, which were also multi-national and enrolled patients with mild-to-moderate AD [13, 14]. An important caveat in the comparison of our data with previous evaluations of APOE-ɛ4 carrier status and treatment response is that the prior analyses were derived from trials of longer duration than the 12-week period covered by our dataset.
Our results are also consistent with larger evaluations of APOE genotype and treatment response that involved other acetylcholinesterase inhibitors, including clinical trials of metrifonate, galantamine, and rivastigmine [22, 24, 25]. In the largest assessment to date, which included 585 patients treated with metrifonate, no interaction was detected between APOE-ɛ4 allele status and response to treatment [24]. Similarly, Aerssens and colleagues reported no effect of APOE-ɛ4 presence or copy number on the rate of cognitive and functional decline in a population of 569 patients treated with galantamine [22]. In a slightly smaller assessment that included 367 patients with mild-to-moderate AD, Farlow and colleagues observed greater cognitive decline among APOE-ɛ4 non-carriers during treatment with either rivastigmine or placebo, yet because the deterioration was observed with both active treatment and placebo, the net result was a comparable treatment response in the APOE-ɛ4 carrier and non-carrier subgroups [25]. Datasets for all of these analyses were derived from pooling of multi-center, double-blind, placebo-controlled studies that enrolled patients with mild-to-moderate AD and evaluated treatment response using ADAS-cog, a global measure of cognition [22, 24, 25].
An intriguing finding in these analyses was the influence of APOE-ɛ4 carrier status on change from baseline to final measurement in ADAS-cog score in the placebo-treated group. Individuals heterozygous or homozygous for the APOE-ɛ4 allele demonstrated an attenuated placebo improvement compared with patients without an APOE-ɛ4 allele. These results are consistent with observations from many previous studies that have suggested that APOE-ɛ4 carriers exhibit a lower placebo response or a greater degree of decline over time in AD clinical trials [31–33] or in observational cohorts [34–36]. There is evidence to suggest that brain structure and function in patients with AD are influenced by APOE genotype, raising the possibility that the lack of improvement and increased rate of decline observed in APOE-ɛ4 carriers in clinical trials is related to underlying differences in disease progression. Investigators have found that carriers of the APOE-ɛ4 allele have more pronounced hippocampal atrophy compared with non-carriers [37, 38]. Moreover, functional magnetic resonance imaging has revealed significant differences in the activity of various brain regions in carriers compared with non-carriers [39].
Another possible explanation for the differences in response to placebo among APOE-ɛ4 carrier groups is a differential impact of repeated ADAS-cog testing. Improvement in scores after repeated administration of cognitive measurement instruments (i.e., practice effects) have been reported among patients with AD [40, 41]. In their recent assessment of amyloid levels and APOE-ɛ4 carrier status in clinically normal older individuals, Mormino and colleagues [42] observed the highest rate of short-term cognitive decline among patients who were APOE-ɛ4+ and had high amyloid-β levels. Our results, together with that of Mormino et al., indicate that if APOE genotype influences practice effects, APOE-ɛ4 non-carriers benefit to a greater extent than do APOE-ɛ4 carriers.
If the findings for the relationship between APOE-ɛ4 carrier status and placebo response are replicated in other datasets, this relationship has the potential to influence clinical trial results. APOE-ɛ4 carriers represent a considerable proportion of the study population (58% to 67%) in AD clinical trials [43]. Differences in distribution of APOE-ɛ4 carriers among treatment groups could have an important impact on the ability to generate a consistent placebo response and to quantify the efficacy of study drug treatment. To minimize this potential source of bias, stratification of patients by APOE-ɛ4 carrier status could be considered for future AD pharmacotherapy trials. In addition, enriching study populations with APOE-ɛ4-positive patients may augment the power to detect treatment differences by minimizing placebo group improvement; however, the potential that the increased burden of screening requirements for such a design would outweigh the benefit must be taken into account [32].
There are several limitations inherent in this analysis of pooled data that have relevance for the interpretation of the results. First, the early termination of two of the trials at a point when fewer than two-thirds of patients had completed the 12-week treatment may have led to an underestimation of the final donepezil and placebo responses. Second, the short duration of treatment precluded an assessment of the long-term effects of genotype on treatment response. MacGowan and colleagues [23] noted that, although their data revealed no short-term effect of APOE-ɛ4 allele status on response to acetylcholinesterase inhibitor therapy, there was the suggestion of an effect over the long-term. It is worth noting, however, that in larger 36- and 52-week studies of donepezil, no such trend was observed [13, 14]. Third, whereas evaluation of treatment response by APOE-ɛ4 genotype was prespecified as a secondary analysis in the protocols of the contributing studies, the trials themselves were powered to evaluate differences in ADAS-cog improvement between the investigational agents and placebo. Lastly, other factors that were not evaluated in this analysis could potentially affect the influence of APOE genotype on treatment response. For example, Morgen and colleagues recently reported an interaction between the APOE-ɛ4 allele and a single-nucleotide polymorphism in the phosphatidylinositol-binding clathrin assembly protein (PICALM) gene in patients with very mild or mild AD dementia [44]. Modifier interactions such as this may underlie the inconsistencies that have been observed from studies evaluating the impact of APOE genotype on acetylcholinesterase inhibitor treatment response.
The current study adds to the growing body of literature indicating that APOE-ɛ4 carrier status does not appreciably affect the magnitude of treatment response to donepezil in patients with mild-to-moderate AD. The detection of a significant variation in response to placebo based on APOE-ɛ4 allele status, however, does merit consideration for future clinical study design.
ACKNOWLEDGMENTS
This study was supported by AbbVie Inc. The authors thank Crystal Murcia, PhD, of The JB Ashtin Group, Inc., for assistance in preparing this manuscript for publication; this support was funded by AbbVie. AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the manuscript for publication.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/14-2589r1).
References
1 | Pericak-Vance MA, Bebout JL, Gaskell PCJr, Yamaoka LH, Hung WY, Alberts MJ, Walker AP, Bartlett RJ, Haynes CA, Welsh KA, Earl NL, Heyman A, Clark CM, Roses AD(1991) Linkage studies in familial Alzheimer disease: Evidence for chromosome 19 linkageAm J Hum Genet48: 10341050 |
2 | Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA(1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset familiesScience261: 921923 |
3 | Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S(1993) Apolipoprotein E polymorphism and Alzheimer’s diseaseLancet342: 697699 |
4 | Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD(1993) Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer diseaseProc Natl Acad Sci U S A90: 19771981 |
5 | Raber J, Huang Y, Ashford JW(2004) APOE genotype accounts for the vast majority of AD risk and AD pathologyNeurobiol Aging25: 641650 |
6 | Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM(1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis ConsortiumJAMA278: 13491356 |
7 | Sando SB, Melquist S, Cannon A, Hutton ML, Sletvold O, Saltvedt I, White LR, Lydersen S, Aasly JO(2008) APOEɛ 4 lowers age at onset and is a high risk factor for Alzheimer’s disease; a case control study from central NorwayBMC Neurol8: 9 |
8 | Singh PP, Singh M, Mastana SS(2006) APOE distribution in world populations with new data from India and the UKAnn Hum Biol33: 279308 |
9 | Sadigh-Eteghad S, Talebi M, Farhoudi M(2012) Association of apolipoprotein E epsilon 4 allele with sporadic late onset Alzheimer‘s disease. A meta-analysisNeurosciences (Riyadh)17: 321326 |
10 | Farlow MR, Lahiri DK, Poirier J, Davignon J, Hui S(1996) Apolipoprotein E genotype and gender influence response to tacrine therapyAnn N Y Acad Sci802: 101110 |
11 | Risner ME, Saunders AM, Altman JFB, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses ADfor the Rosiglitazone in Alzheimer’s Disease Study Group(2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s diseasePharmacogenomics J6: 246254 |
12 | Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman Mfor the Bapineuzumab 201 Clinical Trial Investigators(2009) A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer diseaseNeurology73: 20612070 |
13 | Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm A-L, Zhang R, Haglund A, Subbiah Pand the Donepezil Nordic Study Group(2001) A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate ADNeurology57: 489495 |
14 | Rigaud AS, Traykov L, Latour F, Couderc R, Moulin F, Forette F(2002) Presence or absence of at least one epsilon 4 allele and gender are not predictive for the response to donepezil treatment in Alzheimer’s diseasePharmacogenetics12: 415420 |
15 | Kanaya K, Abe S, Sakai M, Fujii H, Iwamoto T(2010) Changes in cognitive functions of patients with dementia of the Alzheimer type following long-term administration of donepezil hydrochloride: Relating to changes attributable to differences in apolipoprotein E phenotypeGeriatr Gerontol Int10: 2531 |
16 | Choi SH, Kim SY, Na HR, Kim B-K, Yang DW, Kwon JC, Park MY(2008) Effect of APOE genotype on response to donepezil in patients with Alzheimer’s diseaseDement Geriatr Cogn Disord25: 445450 |
17 | Bizzarro A, Marra C, Acciarri A, Valenza A, Tiziano FD, Brahe C, Masullo C(2005) Apolipoprotein E ɛ4 allele differentiates the clinical response to donepezil in Alzheimer’s diseaseDement Geriatr Cogn Disord20: 254261 |
18 | Almkvist O, Jelic V, Amberla K, Hellström-Lindahl E, Meurling L, Nordberg A(2001) Responder characteristics to a single oral dose of cholinesterase inhibitor: A double-blind placebo-controlled study with tacrine in Alzheimer patientsDement Geriatr Cogn Disord12: 2232 |
19 | Sjögren M, Hesse C, Basun H, Köl G, Thostrup H, Kilander L, Marcusson J, Edman Å, Wallin A, Karlsson I, Troell M, Wachtmaister G, Ekdahl A, Olofsson H, Sandström A, Andreasen N, Minthon L, Blennow K(2001) Tacrine and rate of progression in Alzheimer’s disease–relation to APOE allele genotypeJ Neural Transm108: 451458 |
20 | Farlow MR, Lahiri DK, Poirier J, Davignon J, Schneider L, Hui SL(1998) Treatment outcome of tacrine therapy depends on apolipoprotein genotype and gender of the subjects with Alzheimer’s diseaseNeurology50: 669677 |
21 | Poirier J, Delisle M-C, Quirion R, Aubert I, Farlow M, Lahiri D, Hui S, Bertrand P, Nalbantoglu J, Gilfix BM, Gauthier S(1995) Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer diseaseProc Natl Acad Sci U S A92: 1226012264 |
22 | Aerssens J, Raeymaekers P, Lilienfeld S, Geerts H, Konings F, Parys W(2001) APOE genotype: No influence on galantamine treatment efficacy nor on rate of decline in Alzheimer’s diseaseDement Geriatr Cogn Disord12: 6977 |
23 | MacGowan SH, Wilcock GK, Scott M(1998) Effect of gender and apolipoprotein E genotype on response to anticholinesterase therapy in Alzheimer’s diseaseInt J Geriatr Psychiatry13: 625630 |
24 | Farlow MR, Cyrus PA, Nadel A, Lahiri DK, Brashear A, Gulanski B(1999) Metrifonate treatment of AD: Influence of APOE genotypeNeurology53: 20102016 |
25 | Farlow M, Lane R, Kudaravalli S, He Y(2004) Differential qualitative responses to rivastigmine in APOEɛ4 carriers and noncarriersPharmacogenomics J4: 332335 |
26 | Rigaud A-S, Traykov L, Caputo L, Guelfi M-C, Latour F, Couderc R, Moulin F, de Rotrou J, Forette F, Boller F(2000) The apolipoprotein E ɛ4 allele and the response to tacrine therapy in Alzheimer’s diseaseEur J Neurol7: 255258 |
27 | McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM(1984) Clinical diagnosis of Alzheimer’sdisease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services TaskForce on Alzheimer’s DiseaseNeurology34: 939944 |
28 | Gault L, Ritchie C, Robieson W, Pritchett Y, Othman A, Lenz R(2013) Efficacy and safety of the alpha7 agonist ABT-126 in mild-to-moderate Alzheimer’s dementia [abstract]Alzheimers Dement9: P138 |
29 | Haig G, Meier A, Pritchett Y, Hall C, Gault L, Lenz R(2012) Evaluation of the efficacy and safety of the H3 antagonist ABT-288 in mild-to-moderate Alzheimer’s disease [abstract]Alzheimers Dement8: P601P602 |
30 | Marek GJ, Katz DA, Meier A, Greco NIV, Zhang W, Liu W, Lenz RA(2014) Efficacy and safety evaluation of HSD-1 inhibitor ABT-384 in Alzheimer’s diseaseAlzheimers Dement10: S364S373 |
31 | Prins ND, van der Flier WM, Brashear HR, Knol DL, van de Pol LA, Barkhof F, Scheltens P(2013) Predictors of progression from mild cognitive impairment to dementia in the placebo-arm of a clinical trial populationJ Alzheimers Dis36: 7985 |
32 | Kennedy RE, Cutter GR, Schneider LS(2014) Effect of APOE genotype status on targeted clinical trialsoutcomes and efficiency in dementia and mild cognitive impairment resulting from Alzheimer’s diseaseAlzheimers Dement10: 349359 |
33 | Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab Clinical Trial I(2014) Two phase 3 trials of bapineuzumab inmild-to-moderate Alzheimer’s diseaseN Engl J Med370: 322333 |
34 | Ito K, Corrigan B, Zhao Q, French J, Miller R, Soares H, Katz E, Nicholas T, Billing B, Anziano R, Fullerton TAlzheimer’s Disease Neuroimaging I(2011) Disease progression model for cognitive deterioration from Alzheimer’sDisease Neuroimaging Initiative databaseAlzheimers Dement7: 151160 |
35 | Salmon DP, Ferris SH, Thomas RG, Sano M, Cummings JL, Sperling RA, Petersen RC, Aisen PS(2013) Age and apolipoprotein E genotype influence rate of cognitive decline in nondemented elderlyNeuropsychology27: 391401 |
36 | Alegret M, Cuberas-Borros G, Espinosa A, Valero S, Hernandez I, Ruiz A, Becker JT, Rosende-Roca M, Mauleon A, Sotolongo O, Castell-Conesa J, Roca I, Tarraga L, Boada M(2014) Cognitive, genetic, and brain perfusion factorsassociated with four year incidence of Alzheimer’s disease from mild cognitive impairmentJ AlzheimersDis41: 739748 |
37 | Caselli RJ(2012) Phenotypic differences between apolipoprotein E genetic subgroups: Research and clinical implicationsAlzheimers Res Ther4: 20 |
38 | Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW(2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: A retrospective studyLancet Neurol10: 785796 |
39 | Matura S, Prvulovic D, Jurcoane A, Hartmann D, Miller J, Scheibe M, O’Dwyer L, Oertel-Knöchel V, Knöchel C, Reinke B, Karakaya T, Fusser F, Pantel J(2014) Differential effects of the APOE4 genotype on brain structure and functionNeuroimage89: 8191 |
40 | Galasko D, Abramson I, Corey-Bloom J, Thal LJ(1993) Repeated exposure to the Mini-Mental State Examination andthe Information-Memory-Concentration Test results in a practice effect in Alzheimer’s diseaseNeurology43: 15591563 |
41 | Meier A, Buracchio T, Martenyi F, Tang Q, Gault L(2013) Subjects with mild-to-moderate Alzheimer’s disease exhibit evidence of practice effects on repeated Measures of the Alzheimer’s Disease Assessment Scale-Cognitive Subscale and the Mini-Mental Status ExaminationJ Nutr Health Aging17: 818 |
42 | Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, Rentz DM, Johnson KA, Sperling RAAlzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing; Harvard Aging Brain Study(2014) Amyloid and APOEAPOE ɛ4 interact to influence short-term decline in preclinical Alzheimer diseaseNeurology20: 17601767 |
43 | Schneider LS, Sano M(2009) Current Alzheimer’s disease clinical trials: Methods and placebo outcomesAlzheimers Dement5: 388397 |
44 | Morgen K, Ramirez A, Frölich L, Tost H, Plichta MM, Kölsch H, Rakebrandt F, Rienhoff O, Jessen F, PetersO , Jahn H, Luckhaus C, Hüll M, Gertz HJ, Schröder J, Hampel H, Teipel SJ, Pantel J, Heuser I, Wiltfang J, Rüther E, Kornhuber J, Maier W, Meyer-Lindenberg A(2014) Genetic interaction of PICALM and APOE is associated with brain atrophy and cognitive impairment in Alzheimer’s diseaseAlzheimersDement10: S269S276 |
Figures and Tables
Fig.1
Study schematic showing design shared among the three studies that contributed to the dataset. Only patients treated with placebo or donepezil were included in the analyses.
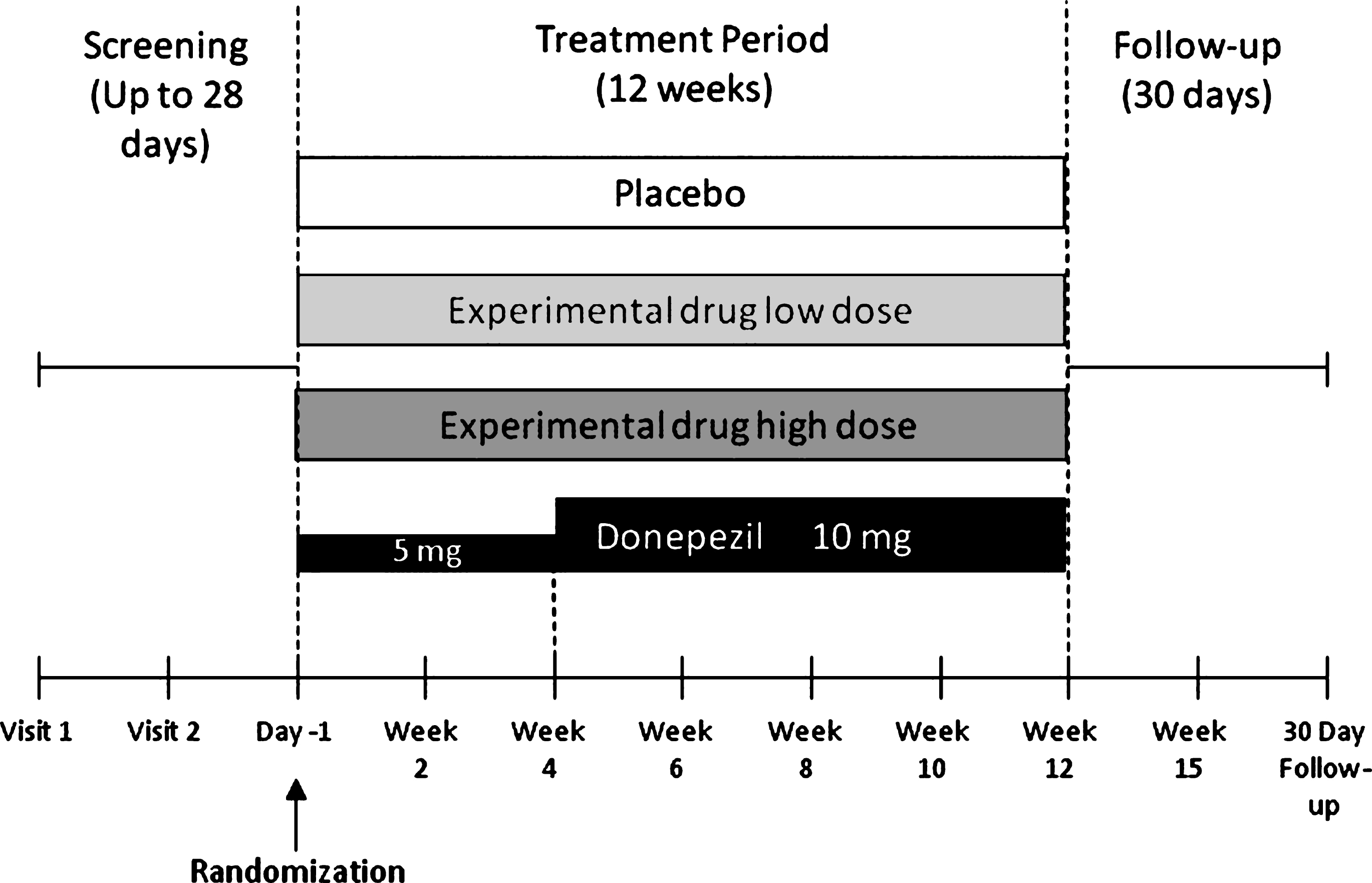
Fig.2
Total score on the 13-item Alzheimer’s Disease Assessment Scale-Cognitive Subscale by apolipoprotein E (APOE)-ɛ4 carrier status. A) Placebo-corrected change from baseline to the final visit with donepezil treatment in the intent-to-treat (ITT) and completer populations. p-values are provided for the comparison of donepezil treatment effect versus placebo. B) Least square (LS) mean change from baseline values for the donepezil and placebo treatment groups (ITT population). Error bars in panel B represent standard error. NS, not significant.
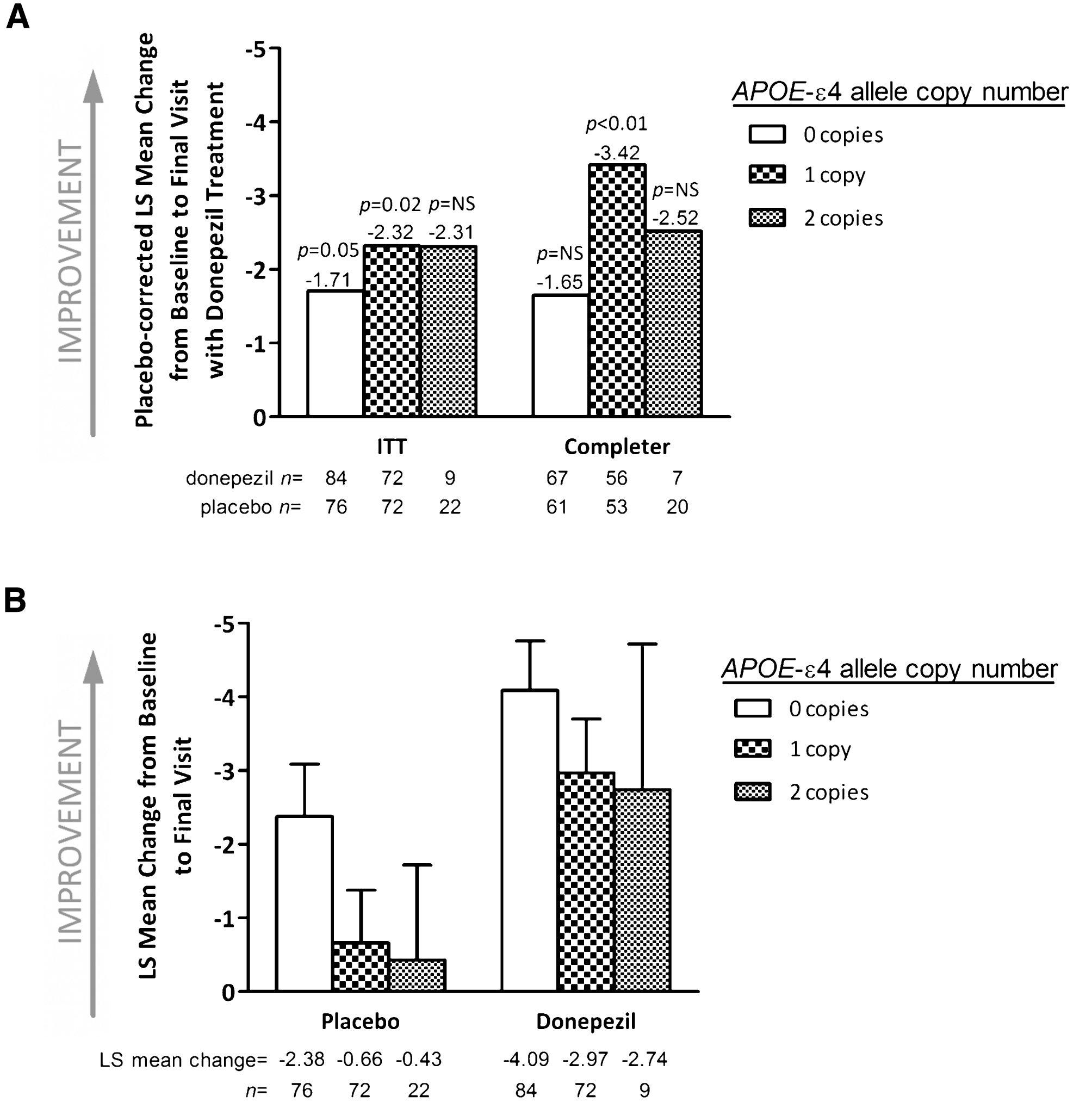
Fig.3
Changes over time in mean Alzheimer’s Disease Assessment Scale-Cognitive Subscale score stratified by treatment group and apolipoprotein E (APOE)-ɛ4 carrier status (intent-to-treat population). Error bars represent standard error.
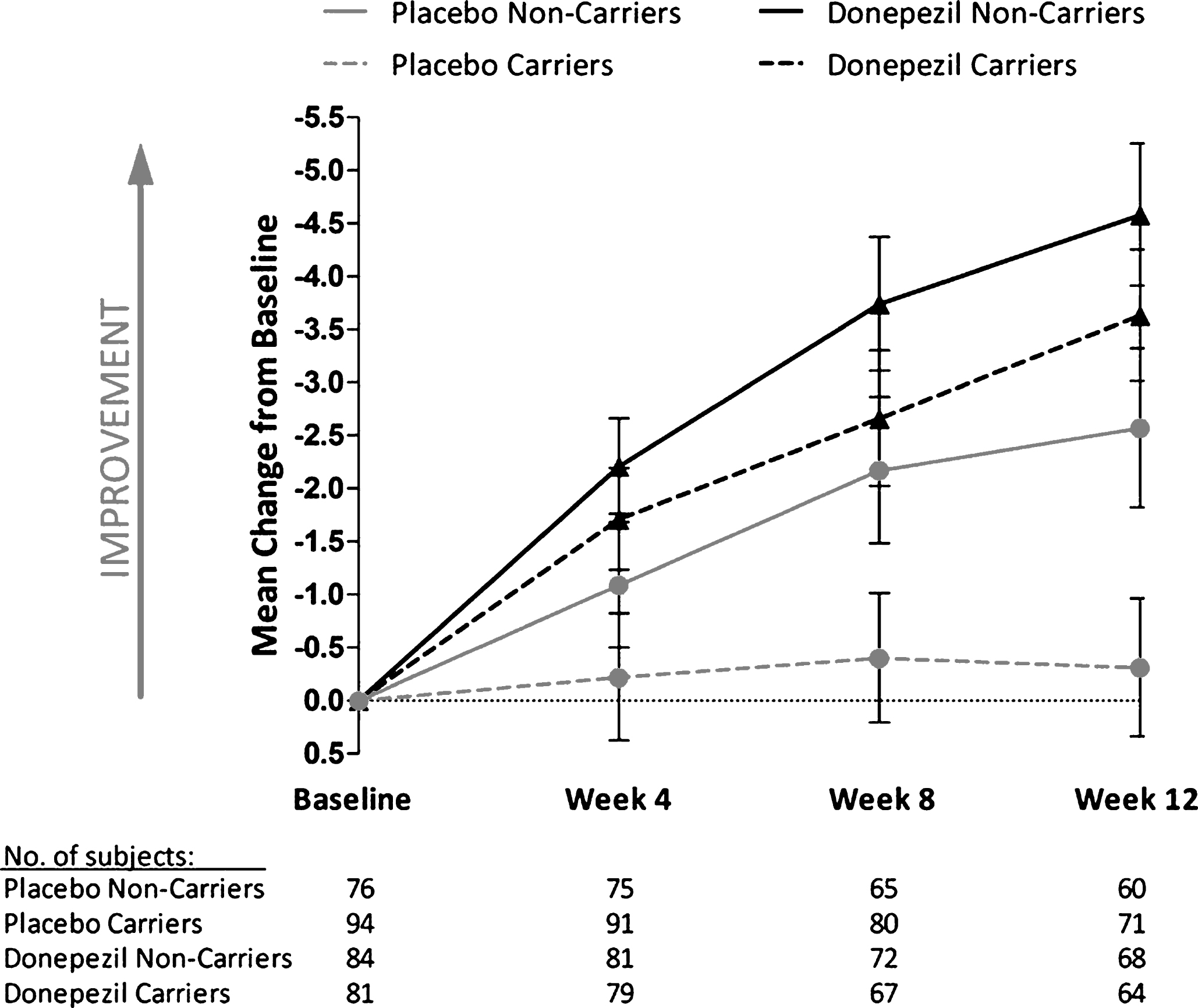
Fig.4
Change from baseline to final assessment in Alzheimer’s Disease Assessment Scale-Cognitive Subscale total score by apolipoprotein E (APOE)-ɛ4 allele copy number. A) Placebo-corrected change from baseline to the final visit with donepezil treatment in the intent-to-treat (ITT) and completer populations. p-values are for the comparison of donepezil treatment effect versus placebo. B) Least square (LS) mean change from baseline values for the donepezil and placebo treatment groups (ITT population). Error bars in panel B represent standard error. NS, not significant.
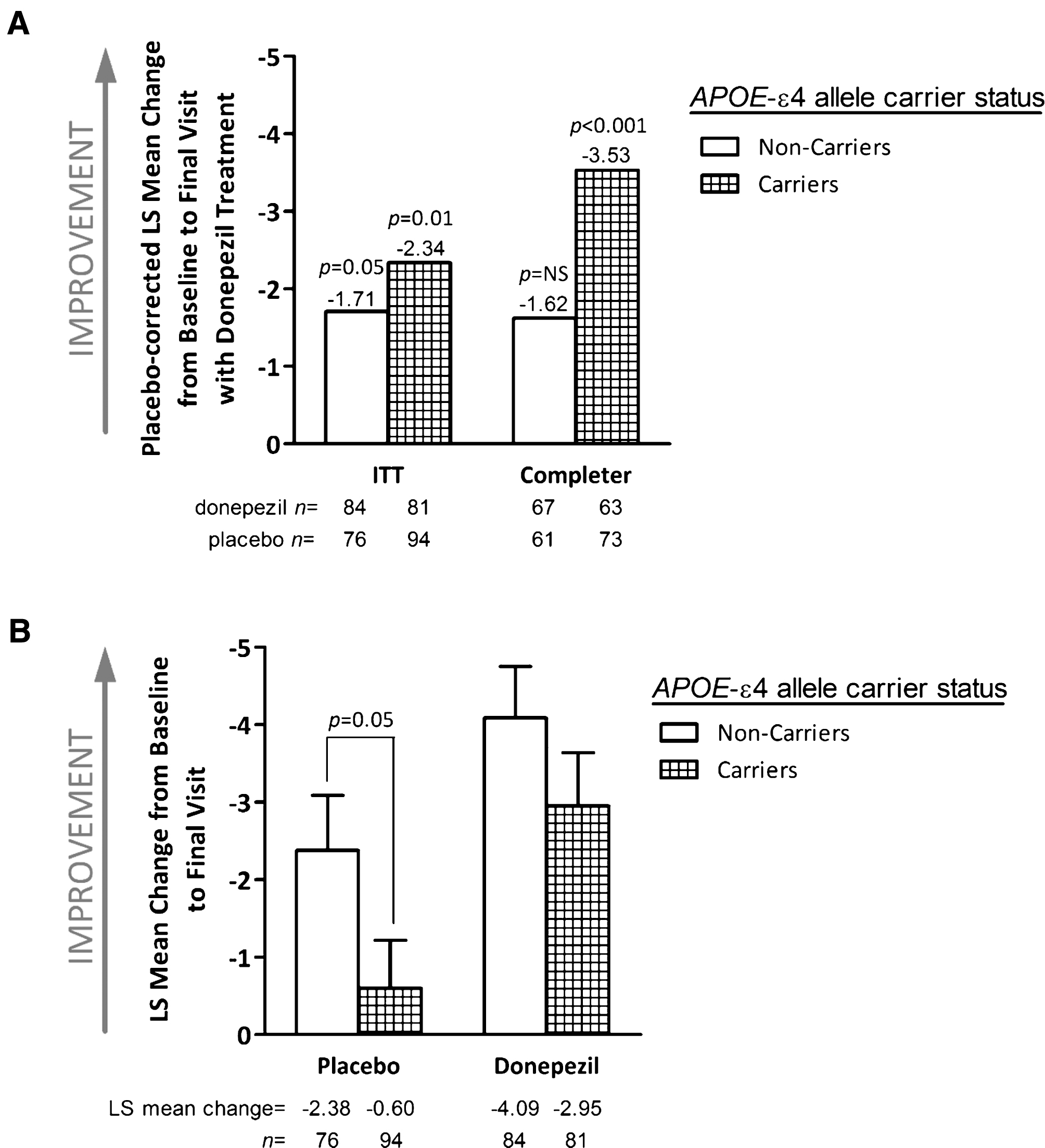
Table 1
Previous studies of APOE-ɛ4 allele status and clinical response to acetylcholinesterase inhibitor treatment
| Study | Sample Size/No. APOE-ɛ4+ | Drug; duration | Efficacy assessment | Results |
| Winblad et al. [13] | 142 / 98 | Donepezil; 52 weeks | GBS scale, MMSE | – |
| Rigaud et al. [14] | 117 / 56 | Donepezil; 36 weeks | ADAS-cog, MMSE, IADL, CGIC | – |
| Choi et al. [16] | 51 / 18 | Donepezil; 48 weeks | ADAS-cog, IADL | > |
| Bizzarro et al. [17] | 41 / 29 | Donepezil; 12 to 16 months | Neuropsychiatric battery | > (in certain tests) |
| Kanaya et al. [15] | 40 / NP | Donepezil; 3 years | ADAS-cog, MMSE | – |
| Aerssens et al. [22] | 569 / NP | Galantamine; 3 to 12 months | ADAS-cog, DAD | – |
| MacGowan et al. [23] | 68 / 49 36 / 20 | Tacrine, Galantamine; 3 months | MMSE | – |
| Farlow et al. [24] | 585 / 349 | Metrifonate; 26 weeks | ADAS-cog, CIBIC-Plus | – |
| Farlow et al. [25] | 367 / 2461 | Rivastigmine; 26 weeks | ADAS-cog | – |
| Sjögren et al. [19] | 145 / 84 | Tacrine; 1 year | CIBIC, MMSE | < (for CIBIC) |
| Farlow et al. [20] | 374 / 245 | Tacrine; 30 weeks | ADAS-cog | < 2 |
| Rigaud et al. [26] | 76 / 33 | Tacrine; variable | ADAS-cog3 | – |
| Poirier et al. [21] | 40 / 22 | Tacrine; 30 weeks | ADAS-cog | < 4 |
| Almkvist et al. [18] | 24 / 14 | Tacrine; single dose | Measures of attention | > |
1Sample size includes both rivastigmine- and placebo-treated patients; 2greater effect size in APOE-ɛ2,3+ versus APOE-ɛ4+ women; 3responsiveness to treatment was defined as a 4-point decrease in ADAS-cog score; 4greater response among APOE-ɛ2,3+ versus APOE-ɛ4+ patients. >, greater response in APOE-ɛ4 carriers; <, Less response in APOE-ɛ4 carriers; — , no significant difference between APOE-ɛ4 carriers and non-carriers; APOE, apolipoprotein E; ADAS-cog, Alzheimer’s Disease Assessment Scale-Cognitive Subscale; CGIC, Clinical Global Impression of Change; CIBIC, Clinician’s Interview Based Impression of Change; CIBIC-Plus, Clinician’s Interview Based Impression of Change with Caregiver Input; DAD, Disability Assessment for Dementia; GBS, Gottfries-Bråne-Steen Rating Scale; IADL, Instrumental Activities of Daily Living; MMSE, Mini-Mental State Examination.
Table 2
Descriptions of consecutive clinical trials by a single sponsor included in the analysis
| Study Characteristic | Trial 1 [28] (NCT00948909) | Trial 2 [29] (NCT01018875) | Trial 3 [30] (NCT01137526) |
| Study drug | ABT-126 | ABT-288 | ABT-384 |
| No. of study sites | 27 | 21 | 30 |
| Countries | Bulgaria, Czech Republic, Slovakia, South Africa, UK, US | Russia, Ukraine | Great Britain, Russia, South Africa, Ukraine |
| Study period | November 2009 to November 2010 | December 2009 to February 2011 | May 2010 to July 2011 |
| Early termination due to futility | No | Yes | Yes |
| Patients randomized to all treatment groups, n | 274 | 242 | 267 |
| Patients randomized to placebo, n (% of total) | 68 (24.8) | 63 (26.0) | 66 (24.7) |
| Patients randomized to donepezil, n (% of total) | 68 (24.8) | 60 (24.8) | 66 (24.7) |
| Completed in all treatment groups, n (% of randomized) | 257 (93.8) | 153 (63.2)1 | 162 (60.7)2 |
| Completed in placebo group, n (% of randomized) | 65 (95.6) | 38 (60.3) | 44 (66.7) |
| Completed in donepezil group, n (% of randomized) | 62 (91.2) | 41 (68.3) | 37 (56.1) |
168 (28.1%) patients discontinued early due to the sponsor decision to terminate the study for futility; 267 (25.1%) patients discontinued early due to the sponsor decision to terminate the study for futility; UK, United Kingdom; US, United States.
Table 3
Subject demographics and baseline characteristics (ITT population)
| Parameter | Placebo (n = 170) | Donepezil (n = 165) | Total (n = 335) |
| Gender, n (%) | |||
| Female | 104 (61.2) | 87 (52.7) | 191 (57.0) |
| Male | 66 (38.8) | 78 (47.3) | 144 (43.0) |
| Age, years1 | 72.2 (8.5) | 71.6 (8.4) | 71.9 (8.5) |
| Non-carriers2 | 71.1 (9.4) | 71.1 (9.2) | |
| Carriers of 1 APOE-ɛ4 copy2 | 73.8 (7.9) | 72.9 (7.5) | |
| Carriers of 2 APOE-ɛ4 copies2 | 70.8 (6.4) | 66.1 (4.8) | |
| Baseline MMSE score1 | 19.2 (4.0) | 19.1 (3.8) | 19.2 (3.9) |
| Baseline 13-item ADAS-cog total score1 | 38.4 (12.9) | 39.9 (13.4) | 39.2 (13.2) |
| ITT population | |||
| Non-carriers | 38.6 (13.4) | 38.8 (13.6) | |
| Carriers of 1 APOE-ɛ4 copy | 37.9 (12.1) | 41.6 (13.4) | |
| Carriers of 2 APOE-ɛ4 copies | 39.6 (14.3) | 37.0 (11.2) | |
| Completer population | |||
| Non-carriers | 37.9 (13.0) | 38.7 (13.9) | |
| Carriers of 1 APOE-ɛ4 copy | 36.4 (11.7) | 42.0 (13.5) | |
| Carriers of 2 APOE-ɛ4 copies | 37.3 (12.4) | 36.8 (11.7) | |
| History of AD medication use, n (%) | |||
| Yes | 62 (36.5) | 59 (35.8) | 121 (36.1) |
| No | 108 (63.5) | 106 (64.2) | 214 (63.9) |
| Years of formal education1 | |||
| Non-carriers | 12.4 (3.3) | 11.3 (3.2) | |
| Carriers | 11.6 (3.1) | 12.0 (3.2) | |
| APOE-ɛ4 carrier status, n (%)3,4 | |||
| Non-carriers | 76 (44.7) | 84 (50.9) | 160 (47.8) |
| Carriers | 94 (55.3) | 81 (49.1) | 175 (52.2) |
| APOE-ɛ4 copy number, n (%)3,4 | |||
| 0 | 76 (44.7) | 84 (50.9) | 160 (47.8) |
| 1 | 72 (42.4) | 72 (43.6) | 144 (43.0) |
| 2 | 22 (12.9) | 9 (5.5) | 31 (9.2) |
Data are for the intent-to-treat (ITT) population unless otherwise noted. 1Data are means (standard deviation); 2Mean values are comparable in the completer population; 3In the completer population, 61, 53, and 20 placebo-treated patients carried 0, 1, and 2 copies of the APOE-ɛ4 allele, respectively, for a total of 61 non-carriers and 73 carriers; 4In the completer population, 67, 56, and 7 donepezil-treated patients carried 0, 1, and 2 copies of the APOE-ɛ4 allele, respectively, for a total of 67 non-carriers and 63 carriers; AD, Alzheimer’s disease; ADAS-cog, Alzheimer’s Disease Assessment Scale-Cognitive Subscale; APOE, apolipoprotein E; MMSE, Mini-Mental State Examination.



