Increased erythrocyte adhesion to VCAM-1 during pulsatile flow: Application of a microfluidic flow adhesion bioassay
Abstract
Sickle cell disease (SCD) is characterized by microvascular occlusion mediated by adhesive interactions of sickle erythrocytes (SSRBCs) to the endothelium. Most in vitro flow adhesion assays measure SSRBC adhesion during continuous flow, although in vivo SSRBC adhesive interactions occur during pulsatile flow. Using a well-plate microfluidic flow adhesion system, we demonstrate that isolated SSRBCs adhere to vascular cell adhesion molecule (VCAM-1) at greater levels during pulsatile versus continuous flow. A significant increase in adhesive interactions was observed between all pulse frequencies 1 Hz to 2 Hz (60–120 beats/min) when compared to non-pulsatile flow. Adhesion of isolated SSRBCs and whole blood during pulsatile flow was unaffected by protein kinase A (PKA) inhibition, and exposure of SSRBCs to pulsatile flow did not affect the intrinsic adhesive properties of SSRBCs. The cell type responsible for increased adhesion of whole blood varied from patient to patient. We conclude that low flow periods of the pulse cycle allow more adhesive interactions between sickle erythrocytes and VCAM-1, and sickle erythrocyte adhesion in the context of whole blood may better reflect physiologic cellular interactions. The microfluidic flow adhesion bioassay used in this study may have applications for clinical assessment of sickle erythrocyte adhesion during pulsatile flow.
1Introduction
The unique adhesive properties of sickle erythrocytes (SSRBCs), first described over 3 decades ago [17, 21], are believed to mediate microvascular occlusion in sickle cell disease (SCD). The adhesive properties of SSRBCs have been well-studied in basic science laboratories [6, 20, 30, 45], but are not yet utilized at the bedside to predict risk of vaso-occlusive (VOC) complications, select therapy, or monitor response to therapy in patients with SCD. An important reason adhesive properties are not assessed clinically is that standardized functional adhesion assays are currently unavailable.
Since SSRBC adhesive properties were first described in static adhesion assays [17, 21], assays to measure SSRBC adhesion have continued to evolve (Table 1). To simulate in vivo flow conditions, laboratory-designed parallel plate flow adhesion (PPFA) assays were utilized to study sickle erythrocyte adhesion under physiologic flow conditions [2, 15, 46]. Although PPFA assays have advanced over the years, their low throughput, longer setup times, lack of standardization, and high blood and reagent consumption have made them impractical tools for clinical testing. Microfluidic-based assays have been used more recently to study SSRBC adhesion [18], and offer the advantage of smaller blood and reagent requirements, shorter set-up time, improved standardization, and a more physiologic microenvironment [7, 14, 40, 48]. As a result, microfluidic-based flow adhesion assays provide a platform for clinical bioassays measuring SSRBC adhesive properties during physiologic flow conditions.
Despite pulsatile flow adaptability most microfluidic flow adhesion assays use flow delivery systems that drive continuous blood flow, similar to the earlier PPFA assays on which most of our understanding of SSRBC adhesive interactions is based. SSRBC adhesive interactions contributing to vaso-occlusion predominate in the region of the post-capillary venule [26, 27, 41, 42], which is still under the influence of arterial pulsations [34, 36]. Additionally, the microvascular blood flow in patients with SCD has been shown to have a more pulsatile or periodic flow pattern compared to unaffected control patients [31, 35]. Pulsatile blood flow has been shown to have specific influences on cell behavior, such as increasing platelet adhesion to immobilized collagen and increasing cell-surface exposure of P-selectins [49]. Therefore, it is plausible that pulsatile blood flow may similarly augment adhesive interactions of SSRBCs and other cellular components of whole blood. The influence of pulsatility on SSRBCs in vitro may provide insight into how in vivo hemodynamics influence SSRBC adhesion.
Sickle cell disease is associated with increased circulating cytokines [10], which increase the surface expression of vascular cell adhesion molecule (VCAM-1) and creates a proadhesive surface for SSRBCs [3, 37]. Very late antigen-4 (VLA-4) or α4β1 integrin is one of the most characterized RBC adhesion molecules [17, 38] which support avid adhesion between SSRBCs and endothelial VCAM-1 [11, 16, 17, 25]. This VLA-4/VCAM-1 interaction can be regulated by protein kinase A (PKA) [22]. PKA plays a critical role in shear-dependent cellular responses [4, 5]; however, the role of PKA in regulating the adhesive state of SSRBCs following exposure to pulsatile shear is not known. The role of PKA in shear dependent cellular processes suggests that variable shear may regulate SSRBC adhesion through a PKA-dependent pathway.
This report describes the first application of a commercial well-plate microfluidic flow adhesion bioassay to study adhesive properties of SSRBCs (both in isolation and in whole blood) during pulsatile flow conditions (Table 1). A standardized substrate of immobilized VCAM-1 was used to control for variable VCAM-1 expression on cultured endothelium. Incorporating pulsatile blood flow may provide more clinically relevant adhesion data. This well-plate microfluidic flow adhesion bioassay can facilitate the study of SSRBC adhesion in the clinical environment to determine the value of this adhesion data in selecting therapy and predicting clinical outcomes.
2Materials and methods
2.1Reagents
Protein kinase A inhibitor (PKA-I) and diamidino-2-phenylindole (DAPI) were obtained from Sigma-Aldrich (St. Louis, MO), vascular cell adhesion molecule (VCAM-1) from R&D Systems (Minneapolis, MN), and anti-CD71 antibody from Beckman Coulter (Miami, FL).
2.2Patients
Peripheral blood was obtained from patients with homozygous SS SCD (n = 15) presenting to the Pediatric Sickle Cell Clinic in steady state. Patients were between 3 to 18 years of age, were not receiving hydroxyurea therapy, and did not undergo transfusion within 3 months from blood collection. Informed parental consent, or patient assent when indicated, was obtained in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board at Wayne State University.
2.3Blood preparation
Blood was drawn by venipuncture into 0.109 M sodium citrate and centrifuged at 150 g for 15 minutes at 25°C to isolate SSRBCs. The SSRBC pellet was separated from the buffy coat and platelet rich plasma. All buffers were pre-warmed to 37°C prior to use. For flow adhesion assays, SSRBCs (1×108 cells/mL) and whole blood (1:2 dilution) were diluted in Hanks balanced salt solution (HBSS) supplemented with 1 mM calcium and 1 mM magnesium, 0.3% bovine serum albumin, phenol red, and 20 mM HEPES, pH = 7.4. For PKA inhibition assays, SSRBCs and whole blood were pre-treated with 30 nM PKA-I for 30 minutes.
2.4Flow adhesion assay
Flow adhesion assays were performed with a commercial well-plate micro-fluidic flow adhesion system, Bioflux 1000Z (Fluxion, San Francisco, CA), described in Fig. 1.
2.4.1Coating
Microfluidic channels were perfused with 0.02 μg/ μL of VCAM-1 at 2 dynes/cm2 for 5 minutes. The 24-well plates were incubated at 37°C for 1 hr. Channels were perfused with HBSS (37°C) at 5 dynes/cm2 for 10 minutes to remove unbound substrate.
2.4.2Pulsatile and non-pulsatile flow conditions
Flow adhesion assays were performed under non-pulsatile (0 Hz) and pulsatile flow conditions (1.0, 1.5, and 2.0 Hz), at a shear stress of 1.0 dyne/cm2 (Fig. 2). For PKA inhibition experiments, isolated SSRBCs and whole blood were pretreated with vehicle or 30 nM of a PKA inhibitor for 30 minutes and perfused through micro-fluidic channels for 1 or 5 minutes respectively at 1dyne/cm2. To assess avidity, adherent SSRBCs were exposed to increasing shear stress conditions (5, 10 and 20 dynes/cm2) for 1 minute and the remaining adherent cells were counted manually.
2.4.3Staining
Adherent cells were fixed with 4% formalin and stained with anti-CD71 antibody (1:20 dilution) overnight at 4°C during static conditions. Adherent cells were washed with 1XPBS and stained with DAPI. Bright field and fluorescent images were overlaid and two independent observers quantified each cell type manually. Adherent cells were identified by morphology and specific staining criteria. Cells that were CD71-/DAPI+ or CD71+/DAPI+ were identified as WBCs if the DAPI staining was consistent with a nuclear staining pattern and cell morphology did not have characteristic erythrocyte features (e.g., biconcave disc). Cells that were CD71+/DAPI- or CD71+/DAPI+ with a diffuse non-nuclear DAPI staining pattern were identified as reticulocytes. Cells that were CD71-/DAPI- were identified as mature erythrocytes.
2.4.4Data acquisition/analysis
Images were acquired with a high resolution CCD camera, in the center of each channel, within the viewing window. Montage imaging software (Molecular Devices, Downington, PA) was used to analyze images. Adherent cells were counted manually.
2.5Static adhesion assay
Isolated SSRBCs (1×108 cells/mL in HBSS) exposed to pulsatile (1.67 Hz) and non-pulsatile (0 Hz) flow conditions were seeded onto 24-well plates pre-coated with 0.02 μg/ μL of VCAM-1, or 0.5% BSA, allowed to adhere for 1 hr at 37°C, aspirated and washed 3 times with HBSS. Adherent cells were counted manually.
2.6Statistical analysis
Patient demographics and outcome measures were assessed with descriptive statistics, including means with standard error of means for skewed continuous variables. Wilcoxon signed rank tests were used to compare isolated SSRBC adhesion to VCAM-1 under non-pulsatile and pulsatile flow conditions and in the presence of PKA inhibition. SPSS version 21.0 (IBM Inc., Chicago IL 2012) was used for statistical analysis. All tests are two-tailed test. P-value <0.05 was considered statistically significant.
3Results
SSRBC adhesion predominates in the post-capillary venules [26, 27, 41–43] at a shear stress of 1dyne/cm2. Shear stress is inversely proportional to SSRBC adhesion [33, 44] thus at lower flow SSRBC adhesion is greater when compared to higher shear stress. Normal resting heart rate for children is variable, ranging from 65–135 bpm from 3 months to 18 years of age [28]. To compare adhesion during non-pulsatile and pulsatile flow conditions at 1dyne/cm2, SSRBC adhesion was measured at 0 Hz and 1.67 Hz (100bpm; average heart rate taken from low and high values), respectively. SSRBCs exhibited a broad range of adhesion to VCAM-1 (1 to 295 SSRBCs/mm2, median = 51) during non-pulsatile flow. Adhesion was significantly increased in the context of pulsatile blood flow compared to non-pulsatile blood flow (Fig. 3A, Table 2). The range of adhesion during pulsatile flow varied from patient-to-patient (22 to 1500 SSRBCs/mm2, median = 237). Adhesion during non-pulsatile compared with adhesion during pulsatile flow was significantly increased from 1.02 to 231-fold, p = 0.001.
There is a direct relationship between increasing pulse frequency and low amplitude flow periods per unit time (Fig. 2). As a result, SSRBC adhesion to VCAM-1 was measured at increasing pulse frequencies. At each pulse frequency tested, SSRBC adhesion increased when compared to non-pulsatile flow of 0 Hz (75.2-fold ± 51.7 at 1.0 Hz; 57.1-fold ± 38.6 at 1.5 Hz; 105.7-fold ± 94.2 at 2.0 Hz;Fig. 3B).
To determine if PKA influences increased SSRBC adhesion to immobilized VCAM-1 during pulsatile flow, adhesion of SSRBCs pretreated with a PKA inhibitor (PKA-I) was compared to untreated SSRBCs under identical flow conditions. Adherent SSRBCs were exposed to increasing shear stress conditions (5, 10 and 20 dynes/cm2) for 1 minute to assess avidity. SSRBC adhesion to VCAM-1 (Fig. 4A) was unaffected by PKA inhibition during pulsatile flow (1.59 ± 0.35 fold change, p = 0.209). Additionally, PKA inhibition did not affect the avidity of SSRBC adhesion to VCAM-1 as measured by adherent SSRBCs still remaining following exposure to increasingly higher shear stresses (p > 0.05 at each shear stress) (Fig. 4B).
To further investigate the adhesive properties of SSRBCs during pulsatile flow, static adhesion of SSRBCs to VCAM-1 was measured following exposure to non-pulsatile versus pulsatile shear (Fig. 4C). There was no significant difference in static adhesion of SSRBCs previously exposed to non-pulsatile versus pulsatile shear (1.3-fold ± 0.18, p = 0.285) (Fig. 4D).
Since adhesion of isolated SSRBCs to VCAM-1 was increased during pulsatile flow, SSRBC adhesion to VCAM-1 was also evaluated in the context of whole blood. Adhesion of whole blood components (SSRBCs, reticulocytes, and white blood cells) increased during pulsatile blood flow compared to non-pulsatile blood flow (3.37-fold ± 0.32; Fig. 5A, Table 3). PKA inhibition had minimal affect on whole blood adhesion during pulsatile flow (1.07-fold ± 0.04; Fig. 5A). Adherent cells were fixed and stained to identify the cell population adhering to VCAM-1 during non-pulsatile and pulsatile flow conditions. The total number of adherent cells increased during pulsatile flow (1.65-fold ± 0.63). This increase was mediated by multiple cell types, and the cell types responsible for the increased adhesion during pulsatile flow varied from patient to patient (Fig. 5B). Reticulocytes and mature SSRBCs accounted for 72% and 25% , respectively, of the adherent cell population during non-pulsatile flow and 60% and 39% , respectively, of adherent cells during pulsatile flow in patient 12 (from 80 cells/mm2 to 86cells/mm2 and from 27 cells/mm2 to 55 cells/mm2, respectively; Fig. 5B). Reticulocytes accounted for 95% of adherent cells during non-pulsatile flow and 97% of the adherent cell population during pulsatile flow conditions in patient 13 (120 cells/mm2 to 293 cells/mm2, respectively) (Fig. 5B). In patient 15 mature SSRBCs and reticulocytes increased during pulsatile flow (335 cells/mm2 to 407 cells/mm2 for mature SSRBCs and 38 cells/mm2 to 96 cells/mm2 for reticulocytes) although mature SSRBCs were the predominant cell type and accounted for 84 and 79% of adherent cells during non-pulsatile and pulsatile flow, respectively (Fig. 5B).
4Discussion
In this report, we utilize a commercially available microfluidic flow adhesion system to measure SSRBC adhesion during pulsatile versus continuous flow. These findings are significant for several reasons. First, SSRBC adhesive properties may be significantly underestimated by in vitro adhesion assays that utilize non-pulsatile flow. Secondly, in vitro SSRBC adhesion during pulsatile flow conditions reflect in vivo adhesive interactions. Finally, the microfluidic flow adhesion assay described in this study can facilitate standardized clinical assessment of SSRBC adhesion.
The average shear SSRBCs experience during pulsatile flow is less than 1 dyne/cm2 since shear varies between a maximum of 1 dyne/cm2 and a minimum approaching 0 dyne/cm2. Previous studies have demonstrated an inverse relationship between SSRBC adhesion and average shear [33, 44], thus we were interested in the relationship between the maximum shear stress on adhesion during constant and pulsatile flow conditions. We observed increased adhesive interactions of isolated SSRBCs (Fig. 3A) and whole blood (Fig. 5A) to VCAM-1 during pulsatile flow versus continuous flow. The cell type responsible for increased adhesion varied from patient to patient in whole blood. For example, reticulocytes comprised the majority of adherent cells during non-pulsatile and pulsatile flow for patients 12 and 13 (Fig. 5B). Patient 13 had the highest reticulocyte count (Table 3) and the greatest number of reticulocytes adhering to VCAM-1 during non-pulsatile and pulsatile flow when compared to patients 12 and 15. Patient 15 had the greatest amount of total cell adhesion during pulsatile flow, comprised almost exclusively of mature SSRBCs and <25% reticulocytes and WBCs. The robust adhesion of mature SSRBCs in patient 15 would not necessarily be predicted by adhesion of isolated SSRBCs (237 SSRBCs/mm2), since isolated SSRBC adhesion was greater in both patients 12 and 13 (378 and 588 SSRBCs/mm2, respectively). These data suggest that adhesion of SSRBCs in the context of whole blood may be considerably different than adhesion of isolated SSRCs, and assessment of whole blood adhesion may provide more insight into the vaso-occlusive risk. These data illustrate the well-described patient-to-patient variability in SSRBC adhesion [20], and suggest a potential role for an adhesion bioassay to distinguish between SCD patients, predict disease severity, and determine subsequent therapy.
VLA-4 on mononuclear cells and reticulocytes [17, 25, 38] mediate greater cell tethering and rolling to VCAM-1 during low shear conditions (0.36 dynes/cm2) [1]. Although a higher surface concentration of VLA-4 gives reticulocytes a greater adhesive advantage during continuous flow [38], reticulocytes are only a small component of whole blood compared to mature erythrocytes. As a result, even slight increases in adhesion of mature erythrocytes during low flow periods of the pulsatile flow cycle can significantly increase the total number of adherent cells [33] (Fig. 6). This is extremely important to note in patients 12 and 15 where mature SSRBC adhesion accounts for 39% and 79% of the adhesion present during pulsatile flow, respectively (Fig. 5B).
Previous studies have shown that shear stress can modulate cellular adhesion to the endothelium via intracellular signaling [39], thus it seemed plausible that pulsatile shear forces could augment cell adhesion by mechanically activating biochemical signals. Inhibition of PKA, a major regulator of SSRBC adhesive properties [20], had no affect on adhesion during pulsatile flow in isolated SSRBCs or whole blood (Fig. 4A and 5A). There was also no difference in the intrinsic adhesive properties of SSRBCs exposed to pulsatile shear compared to continuous shear (Fig. 4D). Thus, it is unlikely that adhesion during pulsatile flow significantly depends on mechanically induced biochemical signals.
The study of SSRBC adhesion during continuous flow has contributed to the growing pipeline of SCD anti-adhesive therapies currently in clinical trials [8, 9, 23, 24, 47]. There are no universally accepted standardized bioassays to measure SSRBC adhesive properties. The current laboratory-specific approaches to assessing adhesive properties are too variable to determine a standardized normal, low, and high range for SSRBC adhesion. For example, in studies where contact between SSRBCs and their corresponding substrates are initiated during continuous flow [19, 20], the degree of patient adhesive interactions may be underestimated. This may be particularly relevant in the context of less avid interactions. For example, P-selectin mediates weak interactions between the endothelium, and both SSRBCs, and WBCs [12, 13, 32]. Pulsatile flow conditions may increase the opportunities for selectin-mediated interactions beyond that observed in previous studies of P-selectin-SSRBC interactions during continuous flow [32]. In studies where SSRBC adhesion is initiated during static conditions and continuous flow subsequently introduced, the degree of adhesion may be overestimated due to the prolonged static contact (approximately 10 minutes) with the adhesive substrate [29]. A standardized clinical bioassay may help providers understand an individual’s risk for vascular occlusion and monitor response to the growing number of anti-adhesive therapies in clinical trials [8, 9, 23, 24, 47]. Studies to determine if clinical predictive value of pulsatile flow adhesion bioassay described in this report are ongoing in our laboratory.
The well-plate based microfluidic flow adhesion bioassay described in this report may provide a platform to incorporate adhesive properties of mature SSRBCs, reticulocytes, as well as WBCs into a clinical bioassay for preclinical anti-adhesive drug testing, and longitudinal assessment of patient response to anti-adhesive therapy. The low sample volume and reagent consumption, automation, high throughput, and decreased assay time (Table 1) make this platform well suited for clinical application when compared to earlier flow adhesion systems. Overall, the well-plate based microfluidic flow adhesion bioassay described here offers a physiologically relevant platform to study adhesion in SCD as well as other areas of cell biology and drug discovery.
Acknowledgements
The authors would like to thank Dr. Xiufeng Gao and Ashley D’Agostino for helping to collect and analyze data, and Dr. Andrew Campbell, MD for his clinical expertise and critique of this manuscript.
References
1 | Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA(1995) The integrin VLA-4 supports tethering and rolling in flow on VCAM-1J Cell Biol128: 12431253 |
2 | Barabino GA, McIntire LV, Eskin SG, Sears DA, Udden M(1987) Endothelial cell interactions with sickle cell, sickle trait, mechanically injured, and normal erythrocytes under controlled flowBlood70: 152157 |
3 | Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM(2000) Activated monocytes in sickle cell disease: Potential role in the activation of vascular endothelium and vaso-occlusionBlood96: 24512459 |
4 | Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H(2002) Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanismAm J Physiol Heart Circ Physiol283: H1819H1828 |
5 | Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H(2002) Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Serby Akt-independent mechanisms: Role of protein kinase AJ Biol Chem277: 33883396 |
6 | Brittain JE, Mlinar KJ, Anderson CS, Orringer EP, Parise LV(2001) Activation of sickle red blood cell adhesion via integrin-associated protein/CD47-induced signal transductionJ Clin Invest107: 15551562 |
7 | Conant CG, Schwartz MA, Beecher JE, Rudoff RC, Ionescu-Zanetti C, Nevill JT(2011) Well plate microfluidic system for investigation of dynamic platelet behavior under variable shear loadsBiotechnol Bioeng108: 29782987 |
8 | De Castro LM, Zennadi R, Jonassaint JC, Batchvarova M, Telen MJ(2012) Effect of propranolol as antiadhesive therapy in sickle cell diseaseClin Transl Sci5: 437444 |
9 | (2011) Deal watch: Pfizer deal for selectin inhibitor highlights potential of glycomimetic drugsNat Rev Drug Discov10: 890 |
10 | Duits AJ, Pieters RC, Saleh AW, van Rosmalen E, Katerberg H, Berend K, Rojer RA(1996) Enhanced levels of soluble VCAM-1 in sickle cell patients and their specific increment during vasoocclusive crisisClin Immunol Immunopathol81: 9698 |
11 | Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR(1990) VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding siteCell60: 577584 |
12 | Etingin OR, Silverstein RL, Hajjar DP(1991) Identification of a monocyte receptor on herpesvirus-infected endothelial cellsProc Natl Acad Sci U S A88: 72007203 |
13 | Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP(1990) Rapid neutrophil adhesion to activated endothelium mediated by GMP-140Nature343: 757760 |
14 | Gomez-Sjoberg R, Leyrat AA, Pirone DM, Chen CS, Quake SR(2007) Versatile, fully automated, microfluidic cell culture systemAnal Chem79: 85578563 |
15 | Grabowski EF(1987) Sickle erythrocytes adhere to endothelial cell monolayers (ECM’s) exposed to flowing bloodProg Clin Biol Res240: 167179 |
16 | Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH(1980) Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severityN Engl J Med302: 992995 |
17 | Hebbel RP, Yamada O, Moldow CF, Jacob HS, White JG, Eaton JW(1980) Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: Possible mechanism for microvascular occlusion in sickle cell diseaseJ Clin Invest65: 154160 |
18 | Higgins JM, Eddington DT, Bhatia SN, Mahadevan L(2007) Sickle cell vasoocclusion and rescue in a microfluidic deviceProc Natl Acad Sci U S A104: 2049620500 |
19 | Hillery CA, Du MC, Wang WC, Scott JP(2000) Hydroxyurea therapy decreases the in vitro adhesion of sickle erythrocytes to thrombospondin and lamininBr J Haematol109: 322327 |
20 | Hines PC, Zen Q, Burney SN, Shea DA, Ataga KI, Orringer EP, Telen MJ, Parise LV(2003) Novel epinephrine and cyclic AMP-mediated activation of BCAM/Lu-dependent sickle (SS) RBC adhesionBlood101: 32813287 |
21 | Hoover R, Rubin R, Wise G, Warren R(1979) Adhesion of normal and sickle erythrocytes to endothelial monolayer culturesBlood54: 872876 |
22 | Humphries MJ, Sheridan J, Mould AP, Newham P(1995) Mechanisms of VCAM-1 and fibronectin binding to integrinalpha 4 beta 1: Implications for integrin function and rational drug designCiba Found Symp189: 177191discussion 191-199 |
23 | Jakubowski JA, Zhou C, Jurcevic S, Winters KJ, Lachno DR, Frelinger AL3rd, Gupta N, Howard J, Payne CD, Mant TG(2013) A phase 1 study of prasugrel in patients with sicklecell disease, Effects on biomarkers ofplatelet activation and coagulationThromb Res75: 614331444 |
24 | Jakubowski JA, Zhou C, Small DS, Winters KJ, Lachno DR, Frelinger AL3rd, Howard J, Mant TG, Jurcevic S, Payne CD(2013) A phase 1 study of prasugrel in patients with sickle cell disease: Pharmacokinetics and effects on ex vivo platelet reactivityBr J Clin Pharmacol75: 14331444 |
25 | Joneckis CC, Ackley RL, Orringer EP, Wayner EA, Parise LV(1993) Integrin alpha 4 beta 1 and glycoprotein IV (CD36) are expressed on circulating reticulocytes in sickle cell anemiaBlood82: 35483555 |
26 | Kaul DK, Fabry ME, Costantini F, Rubin EM, Nagel RL(1995) In vivo demonstration of red cell-endothelial interaction, sickling and altered microvascular response to oxygen in the sickle transgenic mouseJ Clin Invest96: 28452853 |
27 | Kaul DK, Fabry ME, Nagel RL(1989) Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: Pathophysiological implicationsProc Natl Acad Sci U S A86: 33563360 |
28 | Kliegman R, Nelson WE(2007) Nelson textbook of pediatrics18th edSaundersPhiladelphia |
29 | Koo S, Yang Y, Neu B(2013) Poloxamer 188 reduces normal and phosphatidylserine-exposing erythrocyte adhesion to endothelial cells in dextran solutionsColloids Surf B Biointerfaces112C: 446451 |
30 | Lee SP, Cunningham ML, Hines PC, Joneckis CC, Orringer EP, Parise LV(1998) Sickle cell adhesion to laminin: Potential role for the alpha5 chainBlood92: 29512958 |
31 | Lipowsky HH, Sheikh NU, Katz DM(1987) Intravital microscopy of capillary hemodynamics in sickle cell diseaseJ Clin Invest80: 117127 |
32 | Matsui NM, Borsig L, Rosen SD, Yaghmai M, Varki A, Embury SH(2001) P-selectin mediates the adhesion of sickle erythrocytes to the endotheliumBlood98: 19551962 |
33 | Montes RA, Eckman JR, Hsu LL, Wick TM(2002) Sickle erythrocyte adherence to endothelium at low shear: Role of shear stress in propagation of vaso-occlusionAm J Hematol70: 216227 |
34 | Paques M, Baillart O, Genevois O, Gaudric A, Levy BI, Sahel J(2005) Systolodiastolic variations of blood flow during central retinal vein occlusion: Exploration by dynamic angiographyBr J Ophthalmol89: 10361040 |
35 | Rodgers GP, Schechter AN, Noguchi CT, Klein HG, Nienhuis AW, Bonner RF(1984) Periodic microcirculatory flow in patients with sickle-cell diseaseN Engl J Med311: 15341538 |
36 | Santisakultarm TP, Cornelius NR, Nishimura N, Schafer AI, Silver RT, Doerschuk PC, Olbricht WL, Schaffer CB(2012) In vivo two-photon excited fluorescence microscopy reveals cardiac- and respiration-dependent pulsatile blood flow in cortical blood vessels in miceAm J Physiol Heart Circ Physiol302: H1367H1377 |
37 | Setty BN, Stuart MJ(1996) Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: Potential role in sickle cell diseaseBlood88: 23112320 |
38 | Swerlick RA, Eckman JR, Kumar A, Jeitler M, Wick TM(1993) Alpha 4 beta 1-integrin expression on sickle reticulocytes: Vascular cell adhesion molecule-1-dependent binding to endotheliumBlood82: 18911899 |
39 | Takahashi M, Berk BC(1996) Mitogen-activated protein kinase (ERK1/2) activation by shear stress and adhesion in endothelial cells, Essential role for a herbimycin-sensitive kinaseJ Clin Invest98: 26232631 |
40 | Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, Ware RE, Fletcher DA, Lam WA(2012) In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technologyJ Clin Invest122: 408418 |
41 | Turhan A, Jenab P, Bruhns P, Ravetch JV, Coller BS, Frenette PS(2004) Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytesBlood103: 23972400 |
42 | Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS(2002) Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigmProc Natl Acad Sci U S A99: 30473051 |
43 | Turitto VT(1982) Blood viscosity, mass transport, and thrombogenesisProg Hemost Thromb6: 139177 |
44 | Udani M, Zen Q, Cottman M, Leonard N, Jefferson S, Daymont C, Truskey G, Telen MJ(1998) Basal cell adhesion molecule/lutheran protein, The receptor critical for sickle cell adhesion to lamininJ Clin Invest101: 25502558 |
45 | Wagner MC, Eckman JR, Wick TM(2004) Sickle cell adhesion depends on hemodynamics and endothelial activationJ Lab Clin Med144: 260267discussion 227-228 |
46 | Wick TM, Moake JL, Udden MM, Eskin SG, Sears DA, McIntire LV(1987) Unusually large von Willebrand factor multimers increase adhesion of sickle erythrocytes to human endothelial cells under controlled flowJ Clin Invest80: 905910 |
47 | Wun T, Soulieres D, Frelinger AL, Krishnamurti L, Novelli EM, Kutlar A, Ataga KI, Knupp CL, McMahon LE, Strouse JJ, Zhou C, Heath LE, Nwachuku CE, Jakubowski JA, Riesmeyer JS, Winters KJ(2013) A double-blind, randomized, multicenter phase 2 study of prasugrel versus placebo in adult patients with sickle cell diseaseJ Hematol Oncol6: 17 |
48 | Young EW, Beebe DJ(2010) Fundamentals of microfluidic cell culture in controlled microenvironmentsChem Soc Rev39: 10361048 |
49 | Zhao XM, Wu YP, Cai HX, Wei R, Lisman T, Han JJ, Xia ZL, de Groot PG(2008) The influence of the pulsatility of the blood flow on the extent of platelet adhesionThromb Res121: 821825 |
Figures and Tables
Fig.1
Schematic depiction of the microfluidic flow adhesion (FA) system used for this study. Cell adhesion was measured during physiologic flow conditions using a Bioflux 1000Z well plate, micro-fluidic flow adhesion system (Fluxion, San Francisco, CA). User-defined flow conditions, including pulse frequency (0–2 Hz), shear stress (1–20 dynes/cm2), and temperature (37°C), were regulated by an external control unit consisting of an air compressor and electro-pneumatic regulator. Compressed air forced blood samples downward from the input well, through micro-fluidic channels coursing across the bottom surface of the plate, across a common viewing window (350 μm width × 75 μm height) and ultimately up to a corresponding output well. Digital images were acquired with a high resolution CCD camera (QImaging, Canada).
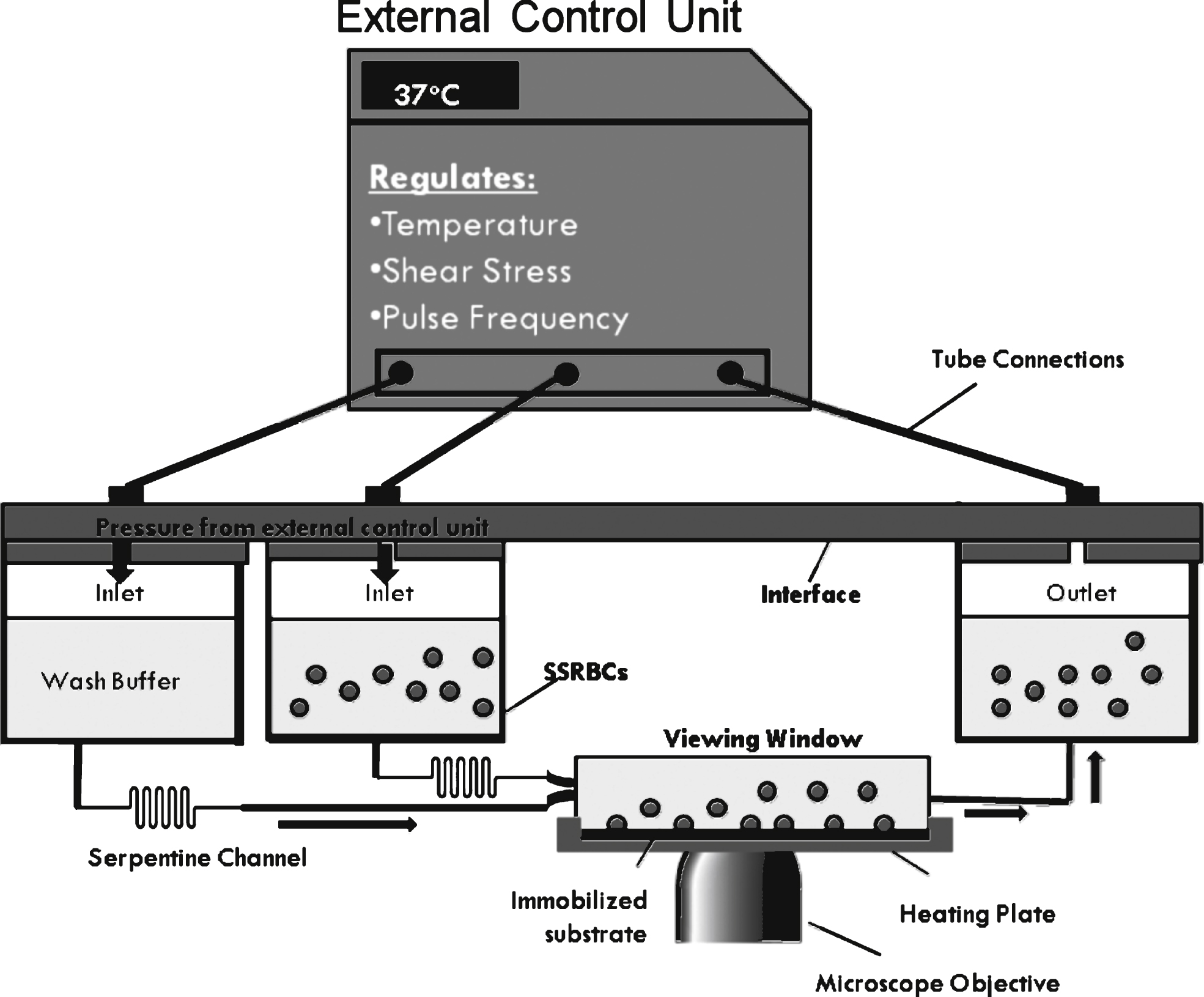
Fig.2
Sample waveforms at maximum amplitude of 1 dyne/cm2. Each cycle is shown within the boxed region. The length of each cycle decreases with increasing frequency. During non-pulsatile flow, a constant pressure is applied to the interface to maintain a user-defined shear, 1 dyne/cm2. To establish pulsatile flow pressure oscillates from 0 to the pressure required to establish 1 dyne/cm2. The grey arrows represent the low shear period of the oscillatory cycle, where we propose many adhesive interactions take place. The number below represents the amount of these low shear periods that occur during the same period. The dashed grey lines represent the slope of the linear region of each oscillation. The slope increases as the frequency increases.
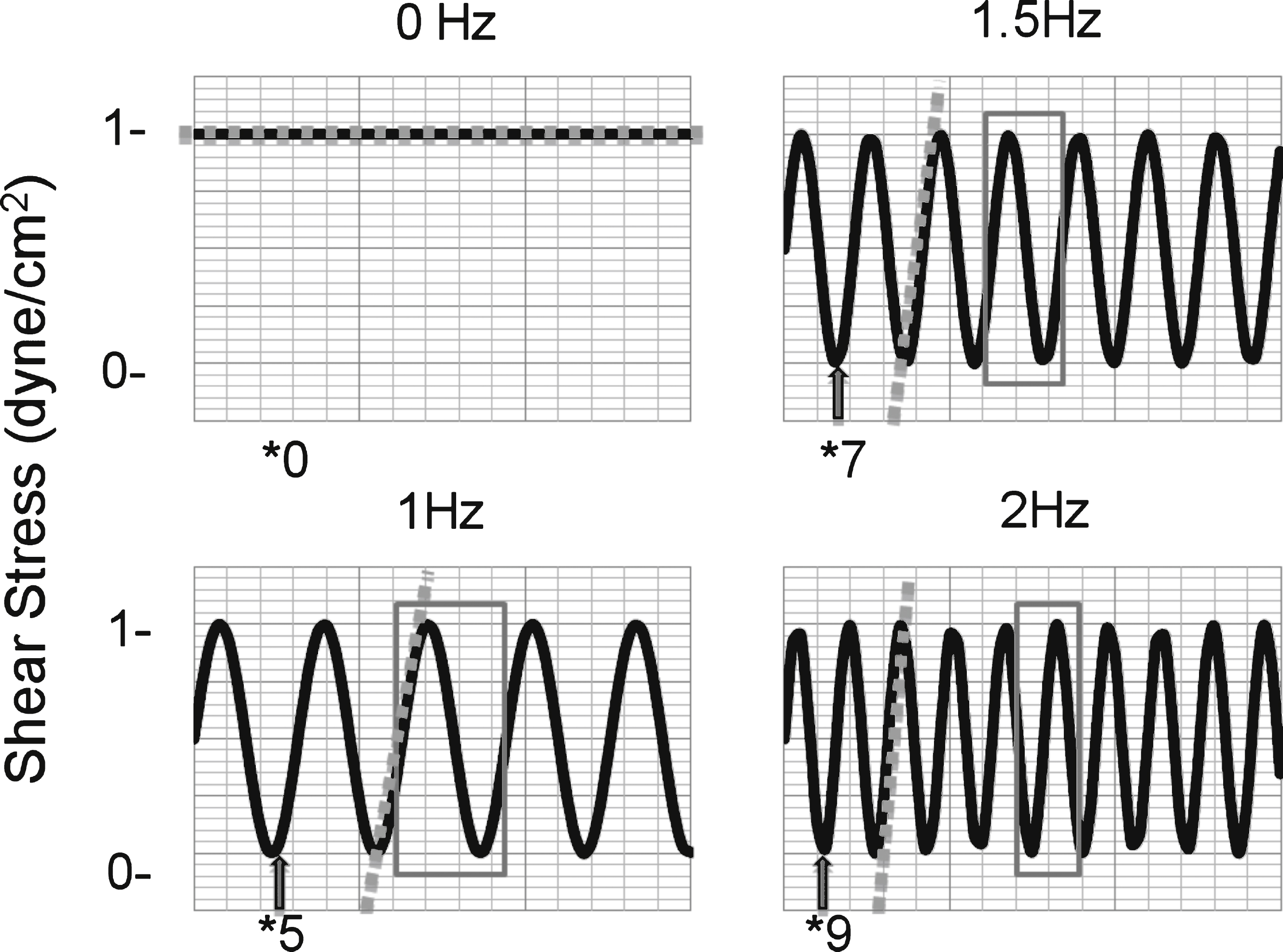
Fig.3
Adhesive interactions of SSRBCs to VCAM-1 increase during pulsatile flow compared to non-pulsatile flow. A) Adhesion of isolated SSRBCs to VCAM-1 (n = 15) was measured during non-pulsatile (0 Hz) and pulsatile (1.67 Hz) flow conditions. A pulse frequency of 1.67 Hz approximates a pulse rate of 100 beats per minute. B) SSRBC adhesion to VCAM-1 was measured during a flow adhesion assay at increasing pulse frequencies (0, 1.0, 1.5, and 2.0 Hz). The dashed line represents the average of the solid lines, which represent separate patients (n = 3).
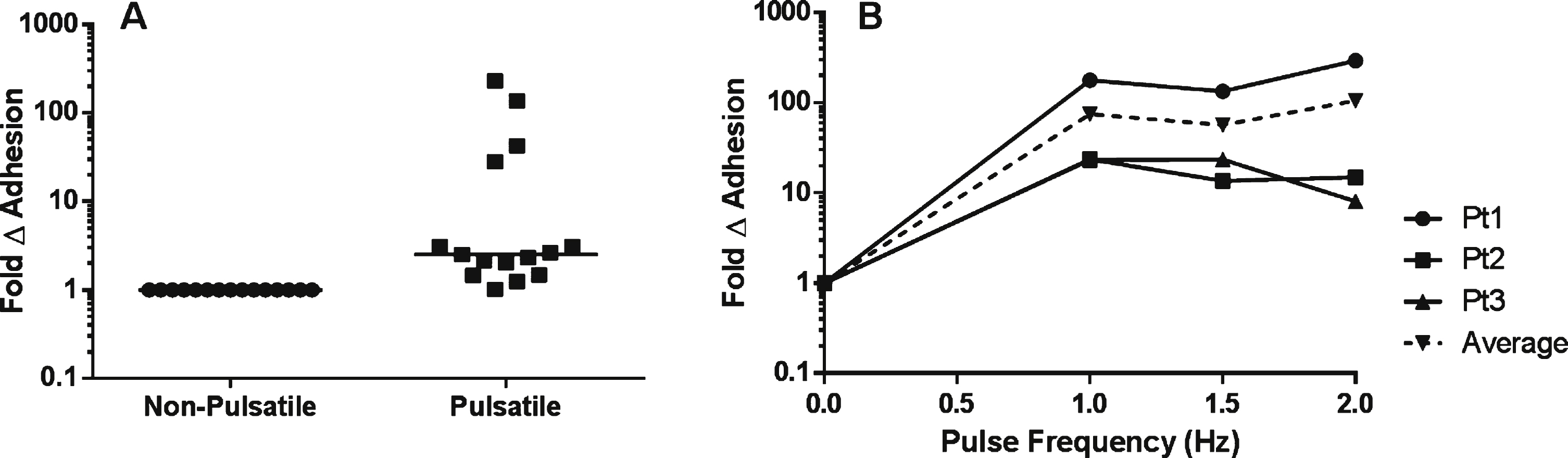
Fig.4
The effects of pulsatile flow on the intrinsic adhesive properties of SSRBCs. A) Adhesion of isolated SSRBCs pretreated with vehicle or 30 nM PKA inhibitor for 30 minutes was measured at 1dyne/cm2 during pulsatile (1.67 Hz) flow conditions. B) Avidity was assessed by exposing adherent SSRBCs to increasing shear conditions (5, 10 and 20 dynes/cm2) and measuring the number of adherent SSRBCs remaining. C) Experimental diagram. Isolated SSRBCs were exposed to either a pulse frequency of 1.67 Hz or non-pulsatile (0 Hz) flow conditions, then allowed to adhere to immobilized VCAM-1 during static conditions in a multi-well plate. Adherent cells were quantified manually. D) Exposure of isolated SSRBCs to pulsatile vs. non-pulsatile flow was assessed during static conditions.
Fig.5
Pulsatile flow conditions increase adhesive interactions in the context of whole blood independent of PKA. A) Adhesion of whole blood pretreated with vehicle or 30 nM PKA inhibitor for 30 minutes was measured at 1dyne/cm2 during non-pulsatile (0 Hz) or pulsatile (1.67 Hz) flow conditions. B) Cellular components of whole blood adhering to VCAM-1 were identified by fixing adherent cells to the micro-fluidic channel and staining with DAPI (nucleic acid) and anti-CD71 antibody (transferrin receptor). C) Representative photomicrographs from Fig. 5B (Patient 15).
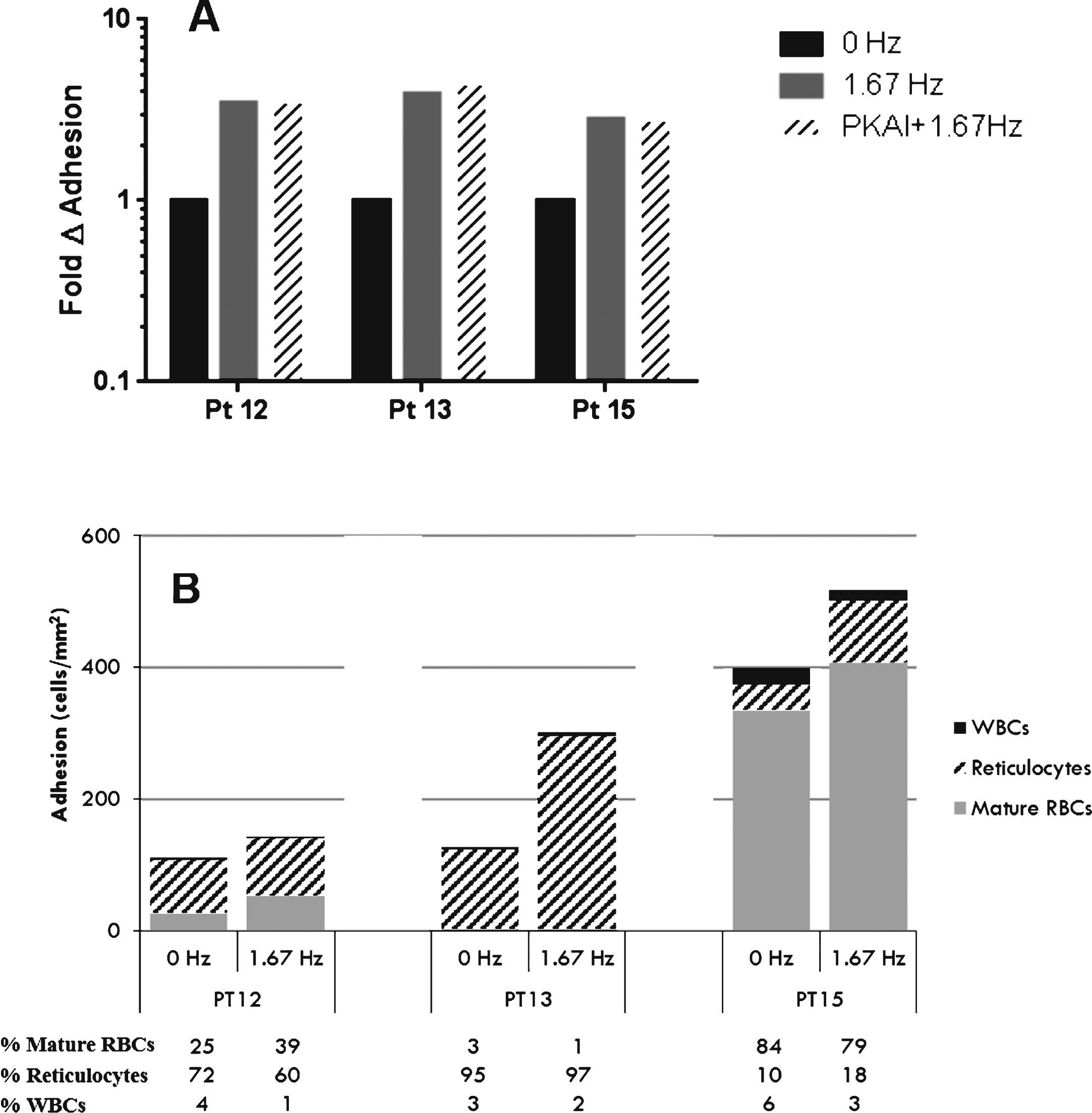
Fig.6
Low shear amplitude during pulsatile flow promotes adhesive interactions between SSRBCs and VCAM-1. A) Blood flow streamlines at the center of the channel during continuous flow and high amplitude blood flow. B) Transitions occurring between high and low shear amplitudes allow blood flow to settle on the bottom surface, thus favoring SSRBC and VCAM-1 interactions. C) At low shear amplitude, blood flow is stalled and blood settles at the bottom surface of the channel. Low flow periods support VLA-4/VCAM-1 interactions. We propose that increased adhesion during pulsatile flow occurs under these conditions.
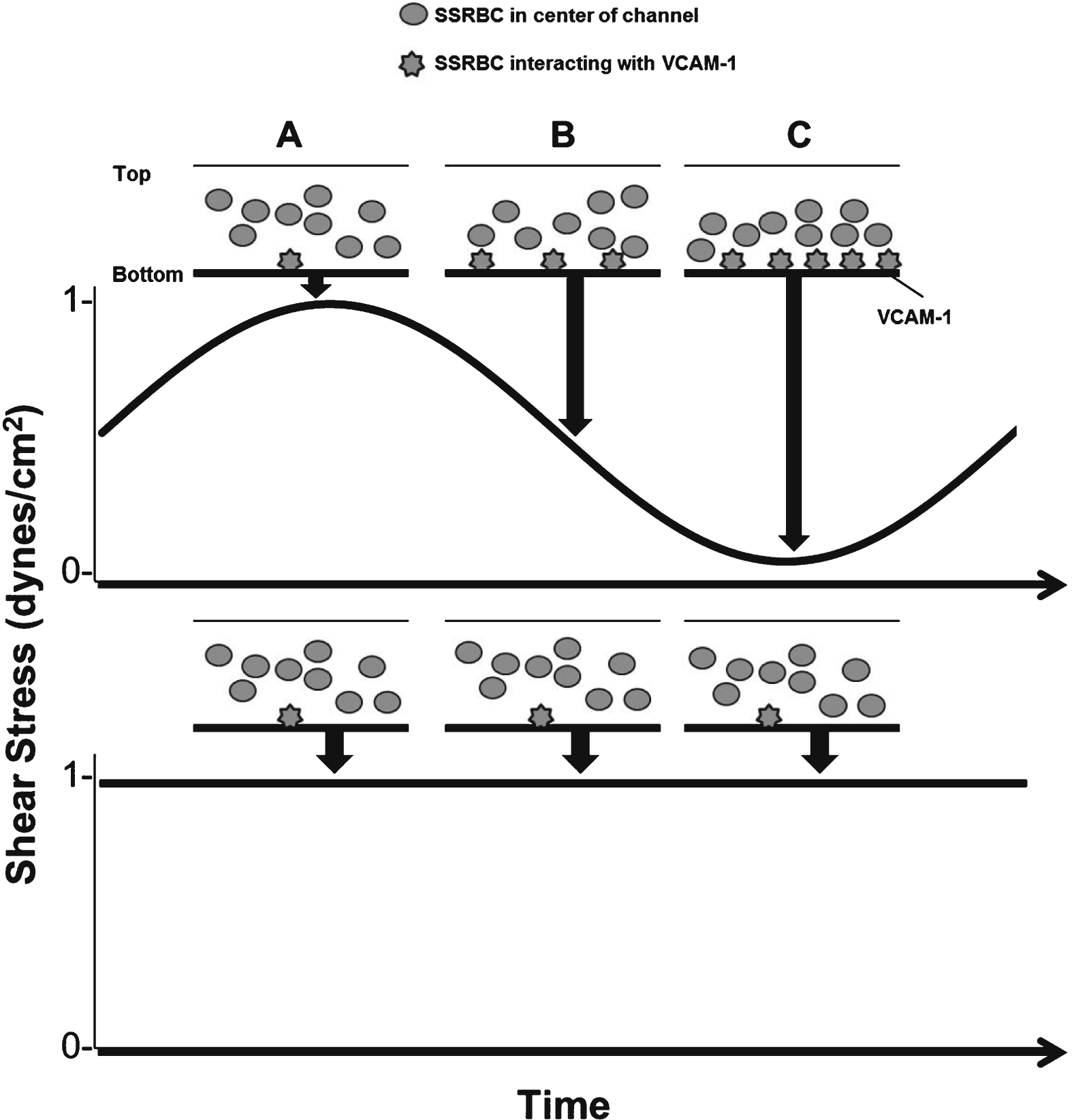
Table 1
Flow Adhesion System Comparison
| Parellel plate flow chamber3,6,53 | Microfluidic chip38,45 | Well plate microfluidics11 | |
| Parallel Experiments | Low | Moderate | High (up to 24) |
| Reagent/Blood consumption | High* | Moderate* | Low* (50 μL-3 mL) |
| Set-Up time | Long | Short | Very short |
| Flow regulation | Peristaltic or syringe pump | Syringe pump | Air compressor and electro-pneumatic regulator |
| Pulsatile flow (Hz) | adaptable | adaptable | 0.1–2.0 |
| Max shear stress (dynes/cm2) | Variable** | Variable** | 200 |
| Channel dimensions (width × height) | Variable*** | Variable*** | 350 μm×75 μm**** |
*Volume depends on size of syringe (typically 10 ml). Sample is added directly to well plate therefore lower sample volumes are allowed. **Maximum shear stress will vary by the dimension of microchannels. ***Microchannels available in various sizes. ****Values correspond to dimensions of the viewing window.
Table 2
Patient demographics and adhesion data for isolated SSRBCs (n = 15)
| Demographics | Adhesion (cells/mm2)* | Fold Change | |||
| Age (Avg = 5.6 ± 2.4 yrs) | Sex (47.7% Female) | 0 Hz | 1.67 Hz | 1.67 Hz vs. 0 Hz | |
| Pt1 | 6 | F | 0.5 | 75 | 138 |
| Pt2 | 9 | M | 1.6 | 379 | 231 |
| Pt3 | 11 | F | 7.1 | 22 | 3.12 |
| Pt4 | 14 | M | 36 | 1007 | 28.36 |
| Pt5 | 11 | M | 26 | 27 | 1.02 |
| Pt6 | 6 | M | 85 | 123 | 1.45 |
| Pt7 | 5 | F | 51 | 77 | 1.49 |
| Pt8 | 18 | F | 225 | 519 | 2.31 |
| Pt9 | 7 | F | 22 | 63 | 2.92 |
| Pt10 | 6 | F | 123 | 233 | 1.89 |
| Pt11 | 10 | M | 38 | 1500 | 39.13 |
| Pt12 | 4 | M | 222 | 588 | 2.65 |
| Pt13 | 3 | M | 295 | 378 | 1.28 |
| Pt14 | 4 | F | 160 | 348 | 2.18 |
| Pt15 | 5 | M | 110 | 237 | 2.15 |
*Adhesion experiments were performed in triplicate. Data in table represents the average of three runs.
Table 3
Whole blood analysis: patient demographics, adhesion, and hematological data
| Demographics | Adhesion (cells/mm2) | Fold Δ Adhesion | Clinical data | ||||||
| Age | Sex | 0 Hz | 1.67 Hz | 0 Hz vs. 1.67 Hz | Reticulocyte (% ) | Hemoglobin (g/dL) | Platelets (×1000/mcL) | White Blood Cells (×1000/mcL) | |
| Pt12 | 4 | M | 408 | 1492 | 3.65 | 16.8 | 8.4 | 619 | 10.8 |
| Pt13 | 3 | M | 689 | 1551 | 2.25 | 25.8 | 7.3 | 251 | 9.1 |
| Pt15 | 5 | M | 167 | 720 | 4.30 | 20.2* | 8.6** | 613** | 7.7** |
*Labs drawn 3 months prior to visit. **Labs drawn 1 month following visit.



