The Role of Lipid Metabolism for Neural Stem Cell Regulation
Abstract
Neural stem/progenitor cells (NSPCs) give rise to billions of cells during development and are critical for proper brain formation. The finding that NSPCs persist throughout adulthood has challenged the view that the brain has poor regenerative abilities and raised hope for stem cell-based regenerative therapies. For decades there has been a strong movement towards understanding the requirements of NSPCs and their regulation, resulting in the discovery of many transcription factors and signaling pathways that can influence NSPC behavior and neurogenesis. However, the role of metabolism for NSPC regulation has only gained attention recently. Lipid metabolism in particular has been shown to influence proliferation and neurogenesis, offering exciting new possible mechanisms of NSPC regulation, as lipids are not only the building blocks of membranes, but can also act as alternative energy sources and signaling entities. Here I review the recent literature examining the role of lipid metabolism for NSPC regulation and neurogenesis.
INTRODUCTION
An adult human brain weighs around one and a half kilograms and contains hundreds of billions of neurons and glia, generated by neural stem/progenitor cells (NSPCs) during embryonic and postnatal development [1, 2]. Disruption of neurogenesis and gliogenesis leads to massive brain dysfunction or death [3, 4]. The findings that NSPCs not only persist throughout adulthood, but that adult neurogenesis plays an important role in learning and memory processes and disease [1, 2, 5, 6], further emphasize how delicately NSPC behavior is regulated. Given the vital importance of brain function, a large amount of research has been dedicated to understanding brain development and NSPC regulation and the many transcription factors and signaling pathways involved in this process [3, 4, 7]. Although most of the signaling cascades eventually influence cellular metabolism to a certain extent, the role of metabolism as an active regulator of stem cell behavior has only recently gained attention. Technical advances to analyze metabolic profiles (reviewed by [8]) have enabled in-depth profiling of various stem cells, and there are now several publications that have identified significant metabolic differences between stem cells and their progeny that point towards a regulatory role of cellular metabolism for stem cell activity. Recently this has been shown to be similar in NSPCs as well [9–12].
The emerging particular role for lipid metabolism to govern proliferation and neurogenesis is the focus of this review. A simple definition of the term “lipids” is challenging as it comprises a large amount of biological substances with different properties, with the most frequently used definition referring to poor solubility in water and good solubility in organic solvents (for a comprehensive resource refer to the “LIPID MAPS Lipidomics Gateway”, http://www.lipidmaps.org). Recent efforts to facilitate communication about lipid research has led to eight lipid categories comprising fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids, and polyketides [13, 14]. Within these groups, a large amount of lipids exists with distinct variability, making the study of lipid metabolism a challenging field. Their pivotal role as a structural component in every plasma membrane/organelle membrane as well as their high energy content make these molecules indispensable for higher organisms. Furthermore, their ability to act as signaling molecules has only begun to be appreciated and provides a source of as yet undiscovered signaling mechanisms [15].
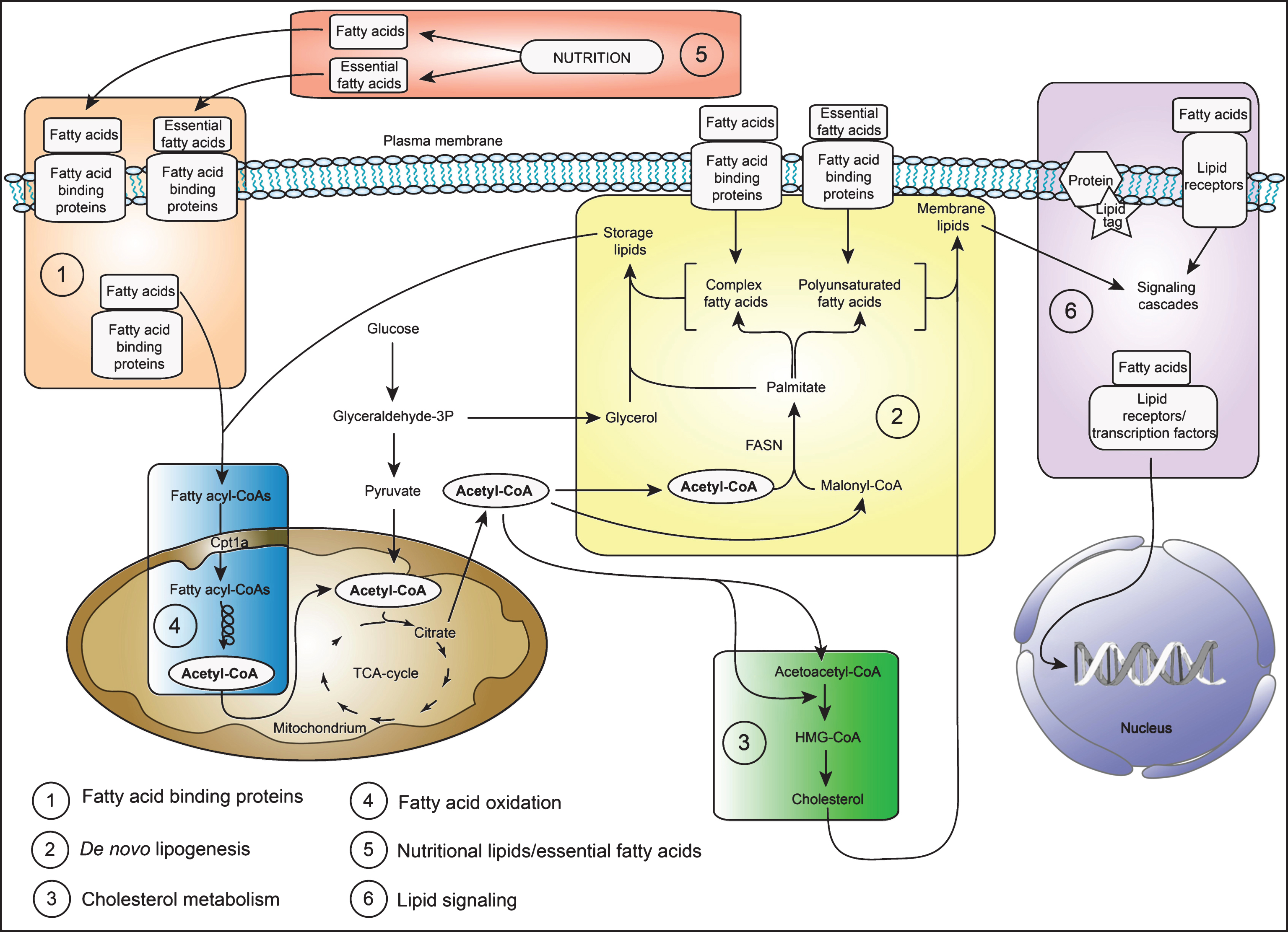
In this review I will discuss the recent literature examining the role of lipid metabolism for NSPC regulation and neurogenesis, with a special focus on adult neurogenesis. To facilitate understanding, an overview of the major lipid metabolic pathways discussed here is shown in a simplified scheme (Fig. 1).
Fig.1
A simplified scheme of the major lipid metabolic pathways in NSPCs. Shown is a schematic drawing of the major lipid metabolic pathways discussed in this review. The numbers indicate the pathways listed in the left hand corner and correspond to the order of appearance in this review. In brief: NSPCs derive their lipids either by taking up nutritional lipids/essential fatty acids (box number 5) through fatty acid binding proteins (box number 1) or synthesize the lipids de novo (box number 2). Lipids, especially cholesterol (box number 3) and complex fatty acids/polyunsaturated fatty acids (box number 2) form important building blocks of all membranes. In addition, lipids can serve as energy substrates and are broken down by fatty acid oxidation in the mitochondria (box number 4) and peroxisomes (not shown). Furthermore, lipids have important signaling functions (box number 6). For more details, please refer to the main text. Abbreviations: TCA (tricarboxylic acid) cycle, CoA (Coenzyme A), FASN (fatty acid synthase), HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A).
FATTY ACID-BINDING PROTEINS IN NSPCs
Most cells obtain their lipids either by synthesizing them de novo via lipogenesis or by taking them up from circulation. As lipids are poorly soluble in aqueous solutions, they need to be transported in a bound form, which in the blood stream usually occurs via albumin, and intracellularly via a class of transport proteins called fatty acid-binding proteins (FABPs) [16]. Three out of ten members of the FABPS, namely FABP3, FABP5 and FABP7 (also called brain lipid binding protein, BLBP) show expression in the developing and adult brain [17, 18]. The expression pattern of these FABPs correlates with specific processes: FABP3 correlates with neurite formation, synapse maturation and myelinogenesis, and FABP5 (and FABP7) are expressed during neuronal cell differentiation and migration. Of special interest in the context of NSPCs is the peak of FABP7 expression, which occurs in mouse around embryonic day 14, the time when neuroepithelial cells transition into radial glial cells [18]. Due to its enrichment in radial glial cells during development but also in the adult brain, FABP7 is now widely used as a NSPC marker protein [19–21]. It has been shown that FABP7 is a direct target of Notch and Pax6 signaling, two signaling pathways highly relevant for NSPCs [22, 23]. The first functional proof for its importance in neurogenesis came from a lineage analysis using a FABP7 (BLBP) promoter-driven Cre recombinase mouse in combination with a Rosa26 reporter mouse, showing that FABP7 is indeed expressed in all radial glial cells throughout the brain and that almost all neurons are derived from these FABP7 expressing cells [24]. Surprisingly, despite complete knockout of FABP7, mice were viable and macroscopic brain structure was normal [25], probably due to compensatory mechanisms even though expression of the other FABPs was unaltered. However, a more detailed analysis by the same group showed a clear decrease in the number of GFAP positive cells in the dentate gyrus (DG) (including radial glia-like cells and astrocytes) and a reduction in proliferation in 4 week-old FABP7 null mice [26, 27], suggesting its functional importance for neurogenesis. Altered emotional behavioral responses and schizophrenic features also have been shown in FABP7 null mice [25, 26]. However, those effects might not only be due to altered neurogenesis: a recent study with FABP7 knockout mice shows that the lack of this protein in all astrocytes throughout the brain led to aberrant dendritic morphology and decreased spine density in neurons, accompanied with altered behavior. Behavioral deficits can be partially rescued by transplantation of wildtype astrocytes, suggesting a wider role of this protein for brain function [28]. To properly distinguish between a general astrocyte influence and altered neurogenesis, conditional knockout mice for FABPs need to be generated allowing for better tissue-specific and temporal control of genedeletion.
DE NOVO LIPOGENESIS IS IMPORTANT FOR NSPC BEHAVIOR
The high expression of FABPs in NSPCs might suggest that they rely entirely on fatty acid uptake from the environment. However, we have recently shown that the alternative way to obtain lipids, namely de novo lipogenesis, is indeed crucial for proliferating NSPCs [29]. In this complex process orchestrated by the multidomain enzyme fatty acid synthase (FASN), 2 carbons coming from malonyl-CoA are sequentially added to an activated acetyl-CoA to form a growing fatty acid chain (for a review of de novo lipogenesis, see [30]). The major product of FASN, palmitic acid, serves as a building block and can be elongated and unsaturated to form more complex lipids. Similar to cancer cells, which produce the majority of their lipids de novo [31], NSPCs also up-regulate the lipogenic pathway during proliferation [29]. The observed high activity level of FASN in proliferating NSPCs is functionally important, as pharmacological and genetic inhibition led to significant decreases in proliferation. Furthermore, inducible conditional knockout of Fasn in adult mice in Nestin-expressing NSPCs led to a drastic reduction in neurogenesis, both in the subventricular zone (SVZ) and in the DG, showing the important role of lipid metabolism for adult neurogenesis [29]. Upregulation of de novo lipogenesis was hindered in quiescent NSPCs through the action of Spot14, a small protein previously implicated in lipid metabolism, which is highly enriched in quiescent NSPCs that can give rise to neurons and astrocytes in vivo [29]. High expression of Spot14 in quiescent NSPCs was recently confirmed in an unbiased single cell RNA-sequencing approach, where Spot14 was among the top 35 genes enriched in quiescent NSPCs [32]. Spot14 acts as a regulator of FASN by reducing Malonyl-CoA levels, which serves as a substrate for FASN, thus lowering substrate availability for de novo lipogenesis [29, 33]. Knocking down Spot14 resulted in increased NSPC activation, further confirming its regulatory role for NSPC behavior. In vivo studies showed that Spot14 positive NSPCs respond dynamically to neurogenic regulators, with increased proliferation to positive stimuli such as running, and decreased amounts of Spot14 positive NSPCs with aging [34]. Furthermore, ablation of the proliferating pool with a cytostatic drug triggered the quiescent Spot14 positive NSPCs to proliferate, showing that the brake on de novo lipogenesis can be released if needed [34]. Interestingly, shortly after the initial description of the role of de novo lipogenesis in adult NSPCs [29], another study was published addressing the role of FASN for exercise-mediated cognitive enhancement [35]. Increased FASN mRNA levels accompanied increased proliferation of NSPCs in the hippocampus of adult mice upon running. Subsequent fatty acid profiling further revealed higher palmitic acid levels, the major product of FASN activity. Moreover, when FASN was inhibited by chronic intracerebroventricular infusion of an inhibitor, exercise-induced increase in proliferation was prevented and the beneficial effects of exercise on cognition weredisrupted [35].
Recently, it has been suggested that the abundance of lipids in niche cells surrounding NSPCs also can directly influence NSPC behavior. Lipids can be stored in so-called lipid droplets, and in Drosophila these lipid droplets were shown to have beneficial effects during oxidative stress [36]. The authors showed that the lipid droplets provided a “safe” storage for membrane polyunsaturated fatty acids (PUFAs) when reactive oxygen species (ROS) levels were high and that protection from lipid peroxidation was necessary for neuroblasts (the NSPCs in Drosophila) to proliferate [36]. How exactly this mechanism is initiated and whether similar mechanisms exist in mammalian neurogenic niches remains to be elucidated. In the mammalian brain, ependymal cells which form part of the neurogenic niche, have been long known for their accumulation of lipid droplets [37] however, the function of those lipid droplets so far has not been studied in detail. A recent report associated an increase in lipid droplets in the ependymal cells with Alzheimer’s disease [38]. In a triple-transgenic mouse model, ependymal cells had a massive increase in lipid droplets, which was accompanied by reduced NSPC proliferation. Such a reduced proliferation was also achieved when lipids were locally increased by intracerebroventricular infusion of oleic acid in wildtype mice, suggesting a direct link between lipid accumulation in niche cells and NSPC regulation [38]. Taken together, FASN dependent de novo lipogenesis in NSPCs and lipid accumulation in niche cells seem to directly regulate NSPC behavior.
CHOLESTEROL METABOLISM IS CRITICAL FOR PROPER NEUROGENESIS
Similar to other lipids, cholesterol is either produced de novo or taken up from the circulation, although in the brain, de novo synthesis is the preferred pathway, especially during development [39]. Cholesterol is a major component of membranes and especially enriched in the brain, as it is a key component of the myelin sheets that isolate the axons [39]. Cholesterol is also crucial for synaptogenesis and manipulation of cholesterol synthesis led to massive brain developmental defects [40]. Despite these massive effects on brain development, only a few studies have addressed the role of cholesterol in NSPCs. An elegant study used conditional ablation of squalene synthase, one of the key enzymes in cholesterol biosynthesis, specifically in NSPCs [41]. This genetic manipulation resulted in reduced brain size caused by massive apoptosis of newborn neurons. The NSPCs however, which were the target of cholesterol synthesis ablation, were largely unaffected. Instead, NSPCs showed an increase in lipid droplets presumably reflecting increased uptake of cholesterol-bearing lipoproteins from external sources. Supporting this hypothesis, NSPCs in mutant embryos upregulated vascular endothelial growth factor (VEGF), which triggers angiogenesis, thereby potentially increasing their supply of cholesterol-rich lipoproteins [41]. Although disturbances in cholesterol metabolism seem to affects newborn neurons to a greater extent than NSPCs, another recent study showed that reduced activity of one of the enzymes in the cholesterol synthesis pathway also disrupts the radial glial fiber scaffold which is provided by NSPCs for the migration of newborn neurons [42]. Furthermore, such reduction in cholesterol biosynthesis affected cell division behavior and led to premature differentiation and loss of self-renewal [42]. Thus, the massive brain developmental defects seen with cholesterol metabolism mutations are likely a result of defects in both NSPCs and newborn neurons. Interestingly, cholesterol supplementation in pregnant mice ameliorated the phenotype in the cholesterol biosynthesis-compromised embryos [42], suggesting that nutritional lipids might significantly influence NSPCs and neurogenesis (see “The role of nutritional lipids on NSPCs and neurogenesis”below).
THE ROLE OF FATTY ACID OXIDATION FOR NSPC REGULATION
Not only the buildup, but also the breakdown of lipids has recently gained attention in the field of stem cell research. The breakdown of lipids occurs via fatty acid oxidation (FAO), mainly in the mitochondria and to a lesser part in the peroxisomes (for very-long-chain fatty acids). This pathway is tightly regulated, as fatty acids cannot enter mitochondria by diffusion but have to be actively transported via a carnitine shuttle system [43]. Oxidation occurs as fatty acids are broken down into acetyl-CoA, which can enter the tricarboxylic acid cycle (TCA) for further energy production, or be used elsewhere, for instance for protein and histone acetylation or as an alternative carbon source [44, 45]. During each oxidation reaction, reduced nicotinamide adenine dinucleotide (NADH), reduced flavin adenine dinucleotide (FADH) and acetyl-CoA is produced, all of which can be used for ATP production. Thus, the energy yield per molecule palmitic acid is more than three times higher than per glucose molecule, making lipids highly efficient energy storingmolecules.
A recent study showed that hematopoietic stem cells (HSCs) rely on FAO for stem cell maintenance and that inhibition of FAO led to an exhaustion of the stem cell pool [46], suggesting an important role of lipid breakdown in HSC biology. However, most classical textbooks state that the brain cannot use fatty acids as an energy source and relies solely on glucose/lactate and ketone bodies. This almost dogmatic view neglects many reports in the early 1970s showing that astrocytes are indeed capable of utilizing fatty acids for energy production and that this also occurs to a certain amount in vivo [47–51]. For a long time it was thought that fatty acids are not penetrating the blood brain barrier, but this has been disproven experimentally [52, 53]. There are other arguments reviewed elsewhere [54], explaining the preferred use of glucose over fatty acids in the brain, but the fact that astrocytes have the necessary machinery to do so raises the question of whether NSPCs might use this metabolic pathway as well. Indeed, a recent study reported that adult NSPCs in the SVZ rely on FAO and that inhibition of FAO decreased proliferation [55]. New data confirm an important role for FAO in NSPCs, suggesting that this pathway is specifically up-regulated in quiescent NSPCs (Knobloch et al. unpublished), and suggest that the two opposite pathways, breakdown and build-up of fatty acids, are important for different stages of NSPC development. Supporting our findings (Knobloch et al. unpublished), many proteins involved in the FAO pathway were enriched in the quiescent NSPC population in single cell RNA sequencing data [32].
Recently, a link has been reported between the clinical association of inborn FAO deficiencies with developmental neuropsychiatric diseases [56]. Inhibition of FAO in the embryonic cortex resulted in a reduction of NSPCs and an increased differentiation, suggesting that FAO might act similarly as in HSCs to maintain the NSPC pool [56]. How exactly FAO exerts this effect remains to beestablished.
THE ROLE OF NUTRITIONAL LIPIDS ON NSPCs AND NEUROGENESIS
As NSPCs can take up circulating lipids, the availability of nutritional lipids might have an impact on NSPC behavior. The high fat diet in the Western world causes increasing health problems, with the resulting obesity linked to heart failures, stroke, diabetes and cancer [57]. A few groups have addressed the overall effects of circulating lipids on NSPCs and neurogenesis. In adult rats, four weeks of high fat diet led to reduced neurogenesis in males without signs of obesity, whereas female rats did gain weight but did not show altered neurogenesis [58]. This somehow surprising gender difference was suggested to be due to altered serum corticosterone levels upon high fat diet only occurring in males [58]. Interestingly, another study showed that high fat diet negatively affected NSPC proliferation and neurogenesis in a mouse strain-specific manner, with the strain more susceptible to obesity being much more affected [59], suggesting that altered physiological factors might cause the decrease in neurogenesis. Another study however, showed that high fat diet only negatively affected neurogenesis during a critical “adolescence” period and had no effect if ingested during adulthood [60]. Likewise, maternal obesity induced by high fat diet before and during pregnancy also negatively affected neurogenesis in the offspring [61]. Although in all these studies there seems to be a general negative effect of high fat diet on neurogenesis, the critical exposure time and the mechanisms of action are not clear.
A different picture regarding the effect of circulating lipids on neurogenesis emerges in the hypothalamus. This particular brain structure senses the availability of peripheral nutrients, such as glucose and circulating lipids and regulates fasting and feeding behavior [62]. It has recently been shown that the hypothalamus also has ongoing neurogenesis throughout adulthood, with the NSPCs being so-called tanycytes that have radial glia-like phenotypes and are located around the third ventricle (reviewed by [63]). The formation of new neurons in the adult hypothalamus has been associated with energy balance [64, 65], and a recent publication demonstrated that these newborn neurons were responsive to metabolic stimuli and increased proliferation upon high-fat diet [66]. Furthermore, upon ablation of hypothalamic neurogenesis by focal irradiation, mice fed a high fed diet gained less weight, probably due to an increased energy expenditure and activity, suggesting that the newborn neurons in the hypothalamus actively contribute to regulation of feeding and activity behavior [66]. Interestingly, other studies published at the same time showed reduced neurogenesis upon high fat diet, however looking at another hypothalamic region [67, 68]. These opposing effects of circulating lipids on two neurogenic areas in the hypothalamus were confirmed in a further study [69]. Taken together, these data suggest that there is indeed a direct effect of circulating lipids on adult NSPCs, but at the same time also reveal that such effects are context- or niche-dependent and need to be studied in much more detail.
THE SPECIAL ROLE OF ESSENTIAL FATTY ACIDS FOR NEUROGENESIS
Mammalians can synthesize most of the different types of lipids de novo with the exceptions of two n-3 and n-6 polyunsaturated fatty acids (PUFAs), also known as omega-3 and omega-6 fatty acids that are essential fatty acids and must be obtained via nutrition. Although in strict terms, there are only two essential fatty acids, alpha-linolenic acid (ALA, an omega-3 fatty acid) and linoleic acid (LA, an omega-6 fatty acid), some of the fatty acids derived from these two precursors, such as for instance, docosahexaenoic acid (DHA) and arachidonic acid (AA), are also often considered as conditionally essential due to their low conversion rate from their precursors [70]. As both DHA and AA have been shown to be required for proper brain development and decrease with age [71–73], the majority of studies on the role of nutritional lipids on NSPCs and neurogenesis have focused on DHA and AA. DHA is produced by microalgae and accumulates in all animals that feed on them, thus is especially enriched in fish. Interestingly, the inclusion of aquatic food into the human diet has been put forward as a driver for human brain evolution, linking DHA to the increase in brain size [74–76]. Besides being very rich in lipids in general, the brain is the organ with the highest amount of DHA [77]. DHA is mainly incorporated into membrane lipids and has been shown to critically influence membrane properties such as fluidity, required for optimal function [78]. Furthermore, it has been shown to be involved in various signaling cascades and inflammatory processes, not only in the brain, but also throughout the body [74, 75]. AA is mainly found in meat and eggs, and is sufficiently available in a Western diet. AA also serves as a precursor for eicosanoids, which in turn are major regulators of the immune system and inflammatory processes [79]. Thus, the complex mechanisms of action of both DHA and AA might exert direct and indirect influence on the brain. Here, only studies directly addressing the role of DHA and AA on NSPCs and neurogenesis are reviewed.
The classical approach to increase the incorporation of DHA and AA into membranes in cells and in the brain is by supplementing DHA/AA in the medium or providing a diet rich in their precursors. Several laboratories chose this approach and the general picture that emerges from these studies is that these two PUFAs are indeed beneficial for neurogenesis both during development and in the adult, promoting neuronal differentiation. The detailed effects however differ between studies, likely due to experimental designs. NSPCs readily incorporated DHA and/or AA that was supplemented in the medium and their membrane lipid profile was more similar to the in vivo situation upon supplementation [80], again pointing towards a need for modifying current culture protocols. Increased availability of PUFAs also increased NSPC proliferation and altered protein localization in lipid rafts [80], however, the consequences of such altered localization was not addressed further. When pregnant female rodents were fed a diet deficient with omega-3 fatty acids, the developing embryos had a smaller cortical plate, hippocampus, and dentate gyrus [81]. NSPCs derived from pups that developed with a maternal diet deficient in omega-3 fatty acid further showed marked and long-lasting effects both on proliferation and differentiation, which were visible up to 40 days in vitro [82]. The number of surviving newborn neurons in vivo was significantly higher with DHA and this was attributed to increased neuronal differentiation and maturation of NSPCs with a reduction in proliferation and cell death, as seen in vitro [83, 84]. In old rats, only 12 weeks of DHA (and eicosapentaenoic acid (EPA)) supplementation was sufficient to reverse age-related decreases in fatty acid receptors and alleviated the decrease in the number of immature neurons, as is usually seen in old animals [85]. Similarly, supplementation of DHA in combination with voluntary exercise, a well known pro-neurogenic stimulus per se, further enhanced synaptic plasticity and cognition, suggesting that pro-neurogenic stimuli might have more room to act when PUFA availability is increased [86]. An elegant approach to artificially increase endogenous DHA in mice was achieved by expression of the C. elegans fat-1 gene that allows conversion of the more abundant omega-6 fatty acids into omega-3 fatty acids [87]. Fat-1 transgenic mice had increased proliferation of NSPCs in vivo [88] and also showed improved cognitive performance. However, this is most probably due to the increase in dendritic spines in CA1 neurons in Fat-1 mice [88], supporting the important role of DHA for neuronal function and health.
Supplementation of the diet specifically with AA was shown to increase NSPC proliferation [89] and an in vitro study suggested that while DHA promotes neuronal differentiation, AA might exert its effects more via maintenance of the NSPC pool [90]. Support for this hypothesis also comes from an AA and DHA supplementation study in aged mice. While AA alone increased proliferation of NSPCs in aged mice, DHA correlated with the number of surviving newborn neurons [91]. Surprisingly, a combination of the two PUFAs did not outperform the single enrichment, which might be explained by the requirement of an “optimal” rate of omega-6 to omega-3 PUFAs, as shown by a recent study addressing the role of too high AA versus DHA ratio [92]. Taken together, the important role of essential fatty acids for proper brain development demonstrates well the key role lipids play for neurogenesis. The evidence that there is requirement for a certain ratio of AA and DHA further illustrates the complexity of this research topic. An isolated view on the role of a certain lipid class might lead to the wrong conclusions and not reflect the complex interplay and competitive behavior of different lipid species. Thus, special care has to be taken when addressing the nutritional influence of lipids on neurogenesis not to oversimplify experimental setups.
THE ROLE OF LIPIDS AS SIGNALING ENTITIES IN NSPCs
As mentioned in the introduction, lipids do not only serve as membrane building blocks or energy molecules but also have a wide variety of signaling functions. They can either directly serve as ligands for membrane and nuclear receptors, such as for instance, for the peroxisome proliferator-activated receptors (PPARs) which in turn act as transcription factors [93]. Lipids can also influence signaling via membrane microdomains called lipid rafts, which form protected areas and can cluster corresponding receptors and ligands. Lipid rafts are highly relevant for signaling cascades involved in stem cell maintenance such as wnt/beta-catenin, EGF- and insulin signaling [94, 95]. Last but not least, lipids can serve as lipid tags on proteins, which alters protein localization and by this influences signaling cascades, as is the case for instance, for sonic hedgehog signaling [96]. Interestingly, a disturbance in membrane lipids and their signaling function has also been implicated in the development of major depression (reviewed by [97]). As defective neurogenesis has been linked to the disease pattern of depression [5], understanding in more detail how lipid signaling is involved in NSPC behavior is of crucial importance.
However, given the complex mechanisms of action and the large variety of signaling lipids, it is beyond the scope of this review to discuss in detail how lipid signaling alters NSPC behavior and neurogenesis (for an in-depth review refer to [15]). That lipid signaling offers a wide and yet underexplored field for neurogenesis is also illustrated by a recent publication: the receptor 1 for lysophosphatidic acid (LPA), a phospholipid with signaling function that had previously been shown to be important for brain development and neurogenesis [98, 99] shows a interesting expression pattern in the adult DG and has been suggested as a novel NSPC marker [100]. As this receptor is a membrane receptor, it provides a novel marker for fluorescence-activated cell sorting (FACS) based NSPC isolation. Furthermore, continuous infusion of LPA over several weeks led to increased survival of newborn neurons, suggesting that lipids involved in signaling indeed have the potential to alter neurogenesis [100].
A LIPID SIGNAL AS A BIOMARKER FOR HUMAN NSPCs?
Given the many studies discussed above that point towards an important role of lipids for NSPC behavior using animal models, the question arises whether this is also true in humans. Interestingly, a specific signal corresponding to NSPCs has been reported in live human brains using proton nuclear magnetic resonance spectroscopy (1H-MRS) [101]. 1H-MRS allows the identification of metabolites in tissues and cells in vitro and in vivo, and a comparison of cultured mouse NSPCs with cultured neurons, astrocytes and oligodendrocytes showed a characteristic signal peak that appeared to be unique for NSPCs. This peak was also significantly higher in cells isolated from the hippocampus of adult mice compared to cortical cells and correlated with levels of neurogenesis. The chemical nature of the peak corresponded to lipids and the peak decreased with inhibition of fatty acid synthesis [101]. Further characterization suggested that it might come from a mix of saturated fatty acids such as palmitic acid and monounsaturated fatty acids such as oleic acid. Excitingly, this peak was also identified and verified with various approaches in adult rats in vivo and in humans [101], offering potentially an non-invasive approach to assess neurogenesis in humans. However, these findings generated concerns, especially regarding technical aspects and the validity of the algorithm used to detect this peak, leading to several comments and responses, all published in Science [102–104]. Others reported that the signal was detectable but not specific to NSPCs and rather a sign for apoptosis [105]. Although this putative lipid peak is intriguing and fits well with the current knowledge of the important role of lipid metabolism in NSPCs, further validation and proof of the methods used are required before this approach might become clinically relevant.
CONCLUSION
Over the last years, evidence for an important role of lipid metabolism in the regulation of neurogenesis has accumulated. Several studies characterized lipid pathways in NSPCs and their progeny and showed their importance through manipulation of key components of these pathways. However, how exactly altered lipid metabolism (due to intracellular changes or due to changes in the extracellular supply) exerts its function needs to be addressed in more detail. By combining technological advances, such as for instance lipidomics (the analysis of the lipid profile by mass spectrometry) and metabolomics (the analysis of thousands of metabolites by mass spectrometry), we will be able to generate a broader and more unbiased picture of what is happening when lipid metabolism is changed. Furthermore, isotopic labeling strategies will allow following the fate of the lipids in more details. The novel technology of mass spectrometry-based imaging of metabolites and lipids in combination with labeling strategies has opened up an especially powerful method of analysis [106, 107]. With detailed information about the spatial location of the studied lipids within a cell or within a tissue, we might gain new insights about potential mechanisms of action. As has happened with conventional imaging, the current resolution and sensitivity limitations of this technique will likely be greatly improved in the future. This might even offer a new way of analysis to address how lipid signaling (for instance through the addition of a lipid tag) changes cellular localization of certain proteins. Last but not least, the promising data on lipid-based signals via 1H-MRS, which correlate with in vivo neurogenesis, might open up completely new avenues to study this important process in a non-invasive way in humans. It is needless to say that such a possibility to study neurogenesis in humans, both in healthy subjects and in patients with neurological diseases, would revolutionize thefield.
CONFLICT OF INTEREST
The author has no conflict of interest to report.
ACKNOWLEDGMENTS
I would like to thank Sebastian Jessberger, Darcie Moore and Ruth Beckervordersandforth for critical comments on the manuscript. My work is supported by the Théodore Ott foundation, Novartis foundation, and the Janggen-Pöhn foundation.
REFERENCES
[1] | Kriegstein A , Alvarez-Buylla A . The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu Rev Neurosci. (2009) ;32: (1):149–84. |
[2] | Taverna E , Götz M , Huttner WB . The Cell Biology of Neurogenesis: Toward an Understanding of the Development and Evolution of the Neocortex. Annu Rev Cell Dev Biol. (2014) ;30: (1):465–502. |
[3] | Pang T , Atefy R , Sheen V . Malformations of cortical development. Neurologist. (2008) ;14: (3):181–91. |
[4] | Sun T , Hevner RF . Growth and folding of the mammalian cerebral cortex: From molecules to malformations. Nat Rev Neurosci. (2014) ;15: (4):217–32. |
[5] | Kang E , Wen Z , Song H , Christian KM , Ming G-L . Adult Neurogenesis and Psychiatric Disorders. Cold Spring Harb Perspect Biol. (2015) ;8: :a019026. |
[6] | Christian KM , Song H , Ming G-L . Functions and Dysfunctions of Adult Hippocampal Neurogenesis. Annu Rev Neurosci. (2014) ;37: (1):243–62. |
[7] | Bond AM , Ming G-L , Song H . Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell. (2015) ;17: (4):385–95. |
[8] | Arnold JM , Choi WT , Sreekumar A , Maletic-Savatic M . Analytical strategies for studying stem cell metabolism. Front Biol. (2015) ;10: (2):141–53. |
[9] | Folmes CDL , Dzeja PP , Nelson TJ , Terzic A . Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. (2012) ;11: (5):596–606. |
[10] | Ito K , Suda T . Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. (2014) ;15: (4):243–56. |
[11] | Homem CCF , Repic M , Knoblich JA . Proliferation control in neural stem and progenitor cells. Nat Rev Neurosci. (2015) ;16: (11):647–59. |
[12] | Knobloch M , Jessberger S . Metabolic control of adult neural stem cell behavior. Front Biol. (2015) ;10: (2):100–6. |
[13] | Fahy E . A comprehensive classification system for lipids. J Lipid Res. (2005) ;46: (5):839–62. |
[14] | Fahy E , Subramaniam S , Murphy RC , Nishijima M , Raetz CRH , Shimizu T , et al. Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res. (2009) ;50: (Suppl):S9–14. |
[15] | Bieberich E . It’s a lipid’s world: Bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochem Res. (2012) ;37: (6):1208–29. |
[16] | Glatz JFC . Lipids and lipid binding proteins: A perfect match. Prostaglandins Leukotrienes & Essential Fatty Acids. (2015) ;93: :45–9. |
[17] | Liu R-Z , Mita R , Beaulieu M , Gao Z , Godbout R . Fatty acid binding proteins in brain development and disease. Int J Dev Biol. (2010) ;54: (8-9):1229–39. |
[18] | Matsumata M , Inada H , Osumi N . Fatty acid binding proteins and the nervous system: Their impact on mental conditions. Neuroscience Research. (2016) ;102: :47–55. |
[19] | Feng L , Hatten ME , Heintz N . Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron. (1994) ;12: (4):895–908. |
[20] | Kurtz A , Zimmer A , Schnütgen F , Brüning G , Spener F , Müller T . The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. (1994) ;120: (9):2637–49. |
[21] | Owada Y , Yoshimoto T , Kondo H . Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J Chem Neuroanat. (1996) ;12: (2):113–22. |
[22] | Anthony TE , Mason HA , Gridley T , Fishell G , Heintz N . Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes & Development. (2005) ;19: (9):1028–33. |
[23] | Arai Y , Funatsu N , Numayama-Tsuruta K , Nomura T , Nakamura S , Osumi N . Role of Fabp7, a downstream gene of Pax6, in the maintenance of neuroepithelial cells during early embryonic development of the rat cortex. Journal of Neuroscience. (2005) ;25: (42):9752–61. |
[24] | Anthony TE , Klein C , Fishell G , Heintz N . Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. (2004) ;41: (6):881–90. |
[25] | Owada Y , Abdelwahab SA , Kitanaka N , Sakagami H , Takano H , Sugitani Y , et al. Altered emotional behavioral responses in mice lacking brain-type fatty acid-binding protein gene. Eur J Neurosci. (2006) ;24: (1):175–87. |
[26] | Watanabe A , Toyota T , Owada Y , Hayashi T , Iwayama Y , Matsumata M , et al. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. Mackay TFC , editor. PLoS Biol. (2007) ;5: (11):e297. |
[27] | Matsumata M , Sakayori N , Maekawa M , Owada Y , Yoshikawa T , Osumi N . The effects of Fabp7 and Fabp5 on postnatal hippocampal neurogenesis in the mouse. Stem Cells. (2012) ;30: (7):1532–43. |
[28] | Ebrahimi M , Yamamoto Y , Sharifi K , Kida H , Kagawa Y , Yasumoto Y , et al. Astrocyte-expressed FABP7 regulates dendritic morphology and excitatory synaptic function of cortical neurons. Glia. (2016) ;64: (1):48–62. |
[29] | Knobloch M , Braun SMG , Zurkirchen L , Schoultz von C , Zamboni N , Araúzo-Bravo MJ , et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. (2013) ;493: (7431):226–30. |
[30] | Ameer F , Scandiuzzi L , Hasnain S , Kalbacher H , Zaidi N . De novo lipogenesis in health and disease. Metabolism. (2014) ;63: (7):895–902. |
[31] | Menendez JA , Lupu R . Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. (2007) ;7: (10):763–77. |
[32] | Shin J , Berg DA , Zhu Y , Shin JY , Song J , Bonaguidi MA , et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell. (2015) :1–14. |
[33] | Colbert CL , Kim C-W , Moon Y-A , Henry L , Palnitkar M , McKean WB , et al. Crystal structure of Spot 14, a modulator of fatty acid synthesis. Proc Natl Acad Sci USA. (2010) 1–6. |
[34] | Knobloch M , Schoultz von C , Zurkirchen L , Braun SMG , Vidmar M , Jessberger S . SPOT14-Positive Neural Stem/Progenitor Cells in the Hippocampus Respond Dynamically to Neurogenic Regulators. Stem Cell Reports. (2014) ;3: (5):735–42. |
[35] | Chorna NE , Santos-Soto IJ , Carballeira NM , Morales JL , la Nuez de J , Cátala-Valentin A , et al. Fatty acid synthase as a factor required for exercise-induced cognitive enhancement and dentate gyrus cellular proliferation. PLoS One. (2013) ;8: (11):e77845. |
[36] | Bailey AP , Koster G , Guillermier C , Hirst EMA , MacRae JI , Lechene CP , et al. Antioxidant role for lipid droplets in a stem cell niche of drosophila. Cell. (2015) ;163: (2):340–53. |
[37] | Sturrock RR , Smart IH . A morphological study of the mouse subependymal layer from embryonic life to old age. J Anat. (1980) ;130: (Pt 2):391–415. |
[38] | Hamilton LK , Dufresne M , Joppé SE , Petryszyn S , Aumont A , Calon F , et al. Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of alzheimer’s disease. Cell Stem Cell. (2015) ;17: (4):397–411. |
[39] | Dietschy JM , Turley SD . Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. (2004) ;45: (8):1375–97. |
[40] | Orth M , Bellosta S . Cholesterol: Its regulation and role in central nervous system disorders. Cholesterol. (2012) ;2012: :292598. |
[41] | Saito K , Dubreuil V , Arai Y , Wilsch-Bräuninger M , Schwudke D , Saher G , et al. Ablation of cholesterol biosynthesis in neural stem cells increases their VEGF expression and angiogenesis but causes neuron apoptosis. Proc Natl Acad Sci USA. (2009) ;106: (20):8350–5. |
[42] | Driver AM , Kratz LE , Kelley RI , Stottmann RW . Altered cholesterol biosynthesis causes precocious neurogenesis in the developing mouse forebrain. Neurobiology of Disease. (2016) ;91: :69–82. |
[43] | Wakil SJ , Abu-Elheiga LA . Fatty acid metabolism: Target for metabolic syndrome. J Lipid Res. (2008) ;50: (Suppl):S138–43. |
[44] | Schoors S , Bruning U , Missiaen R , Queiroz KCS , Borgers G , Elia I , et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. (2015) ;520: (7546):192–7. |
[45] | Carracedo A , Cantley LC , Pandolfi PP . Cancer metabolism: Fatty acid oxidation in the limelight. Nature Reviews Cancer. 13, (2013) ;227: (2013):1–6. |
[46] | Ito K , Carracedo A , Weiss D , Arai F , Ala U , Avigan DE , et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nature Medicine. (2012) ;18: (9):1350–8. |
[47] | Spitzer JJ . CNS and fatty acid metabolism. Physiologist. (1973) ;16: (1):55–68. |
[48] | Warshaw JB , Terry ML . Cellular energy metabolism during fetal development. VI. Fatty acid oxidation by developing brain. Dev Biol. (1976) ;52: (1):161–6. |
[49] | Edmond J , Robbins RA , Bergstrom JD , Cole RA , de Vellis J . Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res. (1987) ;18: (4):551–61. |
[50] | Edmond J . Energy metabolism in developing brain cells. Can J Physiol Pharmacol. (1992) ;70: (Suppl):S118–29. |
[51] | Reichmann H , Maltese WA , DeVivo DC . Enzymes of fatty acid beta-oxidation in developing brain. J Neurochem. (1988) ;51: (2):339–44. |
[52] | Hamilton JA , Brunaldi K . A model for fatty acid transport into the brain. J Mol Neurosci. (2007) ;33: (1):12–7. |
[53] | Mitchell RW , Hatch GM . Prostaglandins, leukotrienes and essential fatty acids. Prostaglandins Leukotrienes & Essential Fatty Acids. (2011) ;85: (5):293–302. |
[54] | Schoenfeld P , Reiser G . Why does brain metabolism not favor burning of fattyacids to provide energy? - Reflections on disadvantages of theuse of free fatty acids as fuel for brain. Journal of Cerebral Blood Flow & Metabolism. (2013) ;33: (10):1493–9. |
[55] | Stoll EA , Makin R , Sweet IR , Trevelyan AJ , Miwa S , Horner PJ , et al. Neural stem cells in the adult subventricular zone oxidize fatty acids to produce energy and support neurogenic activity. Stem Cells. (2015) ;33: (7):2306–19. |
[56] | Xie Z , Jones A , Deeney JT , Hur SK , Bankaitis VA . Inborn errors of long-chain fatty acid b-oxidation link neural stem cell self-renewal to autism. Cell Reports. (2016) ;14: (5):991–9. |
[57] | Deng T , Lyon CJ , Bergin S , Caligiuri MA , Hsueh WA . Obesity, inflammation, and cancer. Annu Rev Pathol. (2016) ;11: :421–49. |
[58] | Lindqvist A , Mohapel P , Bouter B , Frielingsdorf H , Pizzo D , Brundin P , et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. (2006) ;13: (12):1385–8. |
[59] | Hwang IK , Yong Kim Il , Kim DW , Yoo K-Y , Kim YN , Yi SS , et al. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. (2008) ;1241: (C):1–6. |
[60] | Boitard C , Etchamendy N , Sauvant J , Aubert A , Tronel S , Marighetto A , et al. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. (2012) ;22: (11):2095–100. |
[61] | Tozuka Y , Wada E , Wada K . Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. The FASEB Journal. (2009) ;23: (6):1920–34. |
[62] | Morton GJ , Cummings DE , Baskin DG , Barsh GS , Schwartz MW . Central nervous system control of food intake and body weight. Nature. (2006) ;443: (7109):289–95. |
[63] | Lee DA , Blackshaw S . Functional implications of hypothalamic neurogenesis in the adult mammalian brain. International Journal of Developmental Neuroscience. (2012) ;30: (8):615–21. |
[64] | Kokoeva MV . Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science. (2005) ;310: (5748):679–83. |
[65] | Czupryn A , Zhou Y-D , Chen X , McNay D , Anderson MP , Flier JS , et al. Transplanted hypothalamic neurons restore leptin signaling and ameliorate obesity in db/db mice. Science. (2011) ;334: (6059):1133–7. |
[66] | Lee DA , Bedont JL , Pak T , Wang H , Song J , Miranda-Angulo A , et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nature Neuroscience. (2012) ;15: (5):700–2. |
[67] | McNay DEG , Briançon N , Kokoeva MV , Maratos-Flier E , Flier JS . Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. (2012) ;122: (1):142–52. |
[68] | Li J , Tang Y , Cai D . IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nature Cell Biology. (2012) ;14: (10):999–1012. |
[69] | Lee DA , Yoo S , Pak T , Salvatierra J , Velarde E , Aja S , et al. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Front Neurosci. (2014) ;8: :157. |
[70] | Cunnane SC . The conditional nature of the dietary need for polyunsaturates: A proposal to reclassify ‘essential fatty acids’ as ‘conditionally-indispensable’ or “conditionally-dispensable” fatty acids. Br J Nutr. (2000) ;84: (6):803–12. |
[71] | Innis SM . Dietary (n-3) fatty acids and brain development. J Nutr. (2007) ;137: (4):855–9. |
[72] | Bazinet RP , Layé S . Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. (2014) ;15: (12):771–85. |
[73] | Hadley KB , Ryan AS , Forsyth S , Gautier S , Salem N . The essentiality of arachidonic acid in infant development. Nutrients. (2016) ;8: (4). |
[74] | Bradbury J . Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients. (2011) ;3: (12):529–54. |
[75] | Gharami K , Das M , Das S . Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem Int. (2015) ;89: :51–62. |
[76] | Gómez-Pinilla F . Brain foods: The effects of nutrients on brain function. Nat Rev Neurosci. (2008) ;9: (7):568–78. |
[77] | Weiser MJ , Butt CM , Mohajeri MH . Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. (2016) ;8: (2):99. |
[78] | Salem N , Litman B , Kim HY , Gawrisch K . Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. (2001) ;36: (9):945–59. |
[79] | Dennis EA , Norris PC . Eicosanoid storm in infection and inflammation. Nat Rev Immunol. (2015) ;15: (8):511–23. |
[80] | Langelier B , Linard A , Bordat C , Lavialle M , Heberden C . Long chain-polyunsaturated fatty acids modulate membrane phospholipid composition and protein localization in lipid rafts of neural stem cell cultures. J Cell Biochem. (2010) ;110: (6):1356–64. |
[81] | Coti Bertrand P , O’Kusky JR , Innis SM . Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. (2006) ;136: (6):1570–5. |
[82] | Goustard-Langelier B , Koch M , Lavialle M , Heberden C . Rat neural stem cell proliferation and differentiation are durably altered by the in utero polyunsaturated fatty acid supply. The Journal of Nutritional Biochemistry. (2012) 1–8. |
[83] | Kawakita E , Hashimoto M , Shido O . Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. (2006) ;139: (3):991–7. |
[84] | Katakura M , Hashimoto M , Okui T , Shahdat HM , Matsuzaki K , Shido O . Omega-3 polyunsaturated Fatty acids enhance neuronal differentiation in cultured rat neural stem cells. Stem Cells International. (2013) ;2013: :490476. |
[85] | Dyall SC , Michael GJ , Michael-Titus AT . Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res. (2010) ;88: (10):2091–102. |
[86] | Wu A , Ying Z , Gomez-Pinilla F . Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. (2008) ;155: (3):751–9. |
[87] | Kang JX , Wang J , Wu L , Kang ZB . Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature. (2004) ;427: (6974):504. |
[88] | He C , Qu X , Cui L , Wang J , Kang JX . Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. PNAS. (2009) ;106: (27):11370–5. |
[89] | Maekawa M , Takashima N , Matsumata M , Ikegami S , Kontani M , Hara Y , et al. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS One. (2009) ;4: (4):e5085. |
[90] | Sakayori N , Maekawa M , Numayama-Tsuruta K , Katura T , Moriya T , Osumi N . Distinctive effects of arachidonic acid and docosahexaenoic acid on neural stem/progenitor cells. Genes Cells. (2011) ;16: (7):778–90. |
[91] | Tokuda H , Kontani M , Kawashima H , Kiso Y , Shibata H , Osumi N . Differential effect of arachidonic acid and docosahexaenoic acid on age-related decreases in hippocampal neurogenesis. Neuroscience Research. (2014) ;88: :58–66. |
[92] | Sakayori N , Kikkawa T , Tokuda H , Kiryu E , Yoshizaki K , Kawashima H , et al. Maternal dietary imbalance between omega-6 and omega-3 polyunsaturated fatty acids impairs neocortical development via epoxy metabolites. Stem Cells. (2016) ;34: (2):470–82. |
[93] | la Cour Poulsen L , Siersbæk M , Mandrup S . PPARs: Fatty acid sensors controlling metabolism. Seminars in Cell and Developmental Biology. (2012) ;23: (6):631–9. |
[94] | Staubach S , Hanisch F-G . Lipid rafts: Signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics. (2011) ;8: (2):263–77. |
[95] | Lingwood D , Simons K . Lipid rafts as a membrane-organizing principle. Science. (2010) ;327: (5961):46–50. |
[96] | Resh MD . Fatty acylation of proteins: The long and the short of it. Prog Lipid Res. (2016) . |
[097] | Müller CP , Reichel M , Mühle C , Rhein C , Gulbins E , Kornhuber J . Biochimica et biophysica acta. BBA - Molecular and Cell Biology of Lipids. (2015) ;1851: (8):1052–65. |
[98] | Estivill-Torrús G , Llebrez-Zayas P , Matas-Rico E , Santín L , Pedraza C , De Diego I , et al. Absence of LPA1 signaling results in defective cortical development. Cereb Cortex. (2008) ;18: (4):938–50. |
[99] | Matas-Rico E , García-Diaz B , Llebrez-Zayas P , López-Barroso D , Santín L , Pedraza C , et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol Cell Neurosci. (2008) ;39: (3):342–55. |
[100] | Walker TL , Overall RW , Vogler S , Sykes AM , Ruhwald S , Lasse D , et al. Lysophosphatidic acid receptor is a functional marker of adult hippocampal precursor cells. Stem Cell Reports. (2016) ;6: (4):552–65. |
[101] | Manganas LN , Zhang X , Li Y , Hazel RD , Smith SD , Wagshul ME , et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. (2007) ;318: (5852):980–5. |
[102] | Friedman SD . Comment on “Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain”. Science. (2008) ;321: (5889):640. |
[103] | Jansen JFA , Gearhart JD , Bulte JWM . Comment on “Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain”. Science. (2008) ;321: (5889):640. |
[104] | Hoch JC , Maciejewski MW , Gryk MR . Comment on “magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain”. Science. (2008) ;321: (5889):640. |
[105] | Ramm P , Couillard-Despres S , Plötz S , Rivera FJ , Krampert M , Lehner B , et al. A nuclear magnetic resonance biomarker for neural progenitor cells: Is it all neurogenesis? Stem Cells (2009) ;27: (2):420–3. |
[106] | Steinhauser ML , Bailey AP , Senyo SE , Guillermier C , Perlstein TS , Gould AP , et al. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature. (2012) ;481: (7382):516–9. |
[107] | Trim PJ , Snel MF . Small molecule MALDI MS imaging: Current technologies and future challenges. Methods. (2016) ;104: :127–41. |



